"Buy 150mg trileptal fast delivery, medicine in the civil war".
A. Rocko, M.B. B.CH., M.B.B.Ch., Ph.D.
Associate Professor, Texas Tech University Health Sciences Center Paul L. Foster School of Medicine
This raises a question as to whether these are appropriately classified as supplies or would be more appropriately classified as a prosthetic device benefit consistent with the Medicare program definition. If the carrier provides benefits for gradient compression garments, please indicate with a "yes" or "no" whether gradient compression garments for the following body parts are covered: Arms; Hands/Fingers; Feet/Toes; Chest/Breast; Trunk Thorax; Head/Neck; and Abdomen. If the carrier provides benefits for the listed equipment and supplies described in the chart, under what benefit category is it provided? With Questions 5- 5b we sought greater specificity on coverage of gradient compression garments for specific body parts and categorization of those benefits under the insurance policies. Like the responses to Questions 4- 4c, these questions again prompted differing responses that demonstrate that there are some gaps in coverage. However, while these three carrier groups cover garments for all extremities, two carrier groups currently view compression garments for trunk, chest, abdomen, and groin, to be experimental, investigational or unproven and therefore not medically necessary. There is also again a range of responses in how the carrier groups categorize these garments. One carrier even stated that these are covered within the core benefits of the health plan. If the carrier provides benefits for pneumatic compression devices and supplies for some plans only, please specify which plans provide this benefit and which plans exclude this benefit. If the carrier provides benefits for pneumatic compression devices and supplies, please describe any limits, restrictions, or exclusions related to the benefits provided. With Questions 6- 6b we were seeking to determine how carriers cover pneumatic compression devices and appliances. Three of the five carrier groups provide coverage in all plans for Pneumatic Compression Devices or Supplies; Pneumatic Compressor (non-segmental) under code E0650; Pneumatic Compressor without Gradient Pressure (segmental) under code E0651; Pneumatic Compressors with Gradient Pressure (segmental) under code E0652; and Non-segmental Pneumatic Appliances under codes E0655, E0660; E0665; E0666. If coverage is provided for pneumatic compression devices, please indicate with a "yes" or "no" whether pneumatic compression appliances are covered for treatment of the following: Arms, Legs, Chest, Trunk, Head/Neck, and Abdomen. With Questions 5-5b we sought greater specificity on coverage of pneumatic compression devices for specific body parts and any reasons for coverage not being provided. Like the responses to the previous few questions, the differing responses clearly demonstrate that some carrier plans have gaps in coverage. All carriers state that coverage for pneumatic compression devices used to treat the extremities is covered. Two carrier groups indicate that no coverage is provided for pneumatic compression devices that treat the chest, trunk, head, neck, or abdomen because it views such treatment as investigational, experimental, and unproven. Does the carrier provide benefits for the equipment and supplies related to the diagnosis, evaluation and treatment of lymphedema as listed in the chart including Equipment and Supplies, and more specifically Finger/Toe Bandages, Short-Stretch Bandages, Foam/ Padding, Tubular Sleeves, Donning Gloves, and Tape? During the legislative and public meetings there was lots of testimony and discussion about coverage for bandages and the supplies that accompany their use. Question 8 was designed to help pinpoint any gaps in the coverage of the materials commonly used in the 22 treatment of lymphedema. The carrier responses to this question and the sub-questions varied, demonstrating gaps in coverage among the carriers. At a high level, the carriers indicate that coverage for these types of supplies is provided under most, but not all plans. Drilling down to specifics reveals that the carriers use a variety of qualifications or exclusions to coverage. If the carrier provides benefits for complex decongestive therapy, what type of benefit are they considered to be under the policy? Does the carrier provide benefits for any therapies other than complex decongestive therapy for the treatment of lymphedema? If the carrier provides benefits for complex decongestive therapy for some plans only, specify which plans provide this benefit and which plans exclude this benefit. If the carrier provides benefits for complex decongestive therapy, describe any limits, restrictions, or exclusions related to the benefits provided. With these questions we were looking at the level of coverage provided for complex decongestive therapy and the type of therapy carriers considered to be covered within this policy. Each of the carriers responded that benefits for complex decongestive therapy are provided for all plans. Coverage determinations are based on medical necessity and include services that align with company policies and contracts.
This damage can lead to higher risk for the development of cancer and other diseases. Scientists have found that a constant state of lowlevel inflammation-called "chronic inflammation"- can be caused by being overweight or obese (carrying too much body fat). That is because fat cells constantly make inflammatory cytokines (protein molecules that activate immune cells). The belief that white sugar in the diet somehow "feeds" cancer is very common, but the truth is more complicated. All cells, including cancer cells, in the body use sugar (glucose) from the bloodstream for fuel. Blood glucose comes from foods containing carbohydrates, including healthful fruits, vegetables, whole grains, and low-fat dairy products. When there is not enough carbohydrate in the diet, some glucose is even produced by the body from protein-containing foods through a special process. It is excess body fat that has been convincingly linked to greater risk of several types of cancer. Highly refined foods and foods with added sugars, such as sugary drinks and sweets, are also low in fiber and low in nutrients. These foods may also increase insulin resistance, and this has been linked to an increased risk of developing diabetes, heart disease, and overweight and obesity. These comprehensive reviews of cancer research worldwide calculated that approximately 117,000 cancer cases in the United States each year are linked to excess body fat. There are many reasons why people may prefer to eat foods grown organically with fewer pesticide residues. However, studies clearly affirm that consuming a diet rich in fruits and vegetables, whether grown conventionally or organically, is an important part of a diet that lowers overall cancer risk. Fat that accumulates in the abdominal area-lending the body an "apple shape"-is often visceral fat. People with too much visceral fat have been shown to be at greater risk for developing obesity-related diseases and cancer. Another type of fat tissue, subcutaneous fat, is located directly beneath the skin. While these methods are not perfect, they can help people assess whether their weight and waist size fall within the healthy range. Staying within the healthy range throughout life is important for lowering cancer risk. Use a measuring tape and follow these easy steps: Place a tape measure around the waist above the tip of the hipbone. The word "phytochemical" means a naturally occurring plant (phyto, in Greek) chemical. Phytochemicals provide a plant with color, aroma, and flavor as well as protection from infection and predators. The colors, fragrances, and taste of the plant hint at the phytochemicals it contains. In the human diet, some phytochemicals work together to protect the body from cancer and other diseases. Preventing this type of damage might help protect us from cancer and other diseases. The best way to provide the body with phytochemicals is to eat a balanced diet that includes whole grains, legumes, nuts, seeds, and a variety of colorful fruits and vegetables. Avoid foods and drinks that promote weight gain: Consume energy-dense foods sparingly (high calories for amount and few nutrients). Bake until the flesh is tender when pierced, roughly 45 to 90 minutes (depending on size). While the squash is cooling, in a large, heavy pan heat the canola oil over medium-high heat. Per serving: 103 calories, 3 g total fat (<1 g saturated fat), 18 g carbohydrate, 3 g protein, 3 g dietary fiber, 330 mg sodium.
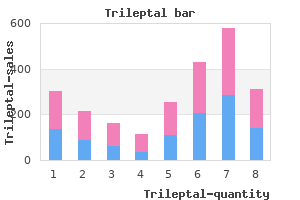
There was no statistically significant difference between the treatment groups with respect to pain during injection (4. Based on the phentolamine dose to which responses were observed, 240 participants were randomized in a crossover design to active treatment versus placebo. Efficacy results were reported only for the 172 men who received at least one dose of active drug and placebo. Obesity, hypertension, and hypercholesterolemia were the most commonly reported underlying diseases. Study Quality and Reporting None of the studies reported the source of pharmaceutical funding. Study withdrawals, drop-outs or participants lost to followup were reported in all trials. Subjects were monitored by RigiScan in the clinic and at home for a total of 6 hours. The number of subjects with improved erections following administration of placebo was not reported. Patients were kept under observation until 24 hours after the dose administration. A greater than two-fold increase in the duration of base rigidity 60 percent, compared with placebo, was reported in 82 percent of subjects receiving the 4 mg dose and 84 percent of patients receiving the 6 mg dose. Two participants experienced extreme nausea and hypotension, with one transiently losing consciousness after the 1. Eleven out of the 12 subjects exceeded a change of 1cm in circumference after injection). Quantitative Synthesis No meta-analysis was performed due to the clinical heterogeneity with regard to intervention types. Of five studies, four assessed clinically relevant efficacy outcome such as home sexual intercourse success 299,300,302,304 and one trial reported on whether in-clinic erections were judged sufficient for intercourse. Of these six trials,299-304 two were cross-over design (n=345; range: 111-234 participants) and four were parallel design (n=1726, range: 60-996 participants). Vascular disease and diabetes were the most commonly reported underlying diseases. Participant withdrawals, drop-outs or lost to followup were reported in all trials and ranged from 7 percent to 42 percent. The majority of the trials were considered to be of low quality as assessed by the Jadad scale. All six trials reported data on penile or urogenital pain and three trials reported results on prolonged erections or priapism/fibrosis. Qualitative Synthesis Summary of qualitative synthesis for this section in presented also in Tables 17-19. In the first trial, compared with men in placebo group, alprostadil-treated men had an increased frequency of penile pain (3. No cases of prolonged erection, priapism or fibrosis were observed in either treatment group. There were no cases of priapism or fibrosis, or urinary tract infection in either treatment group. Pooled clinical efficacy results were presented for treatment groups, namely the proportion of men during the study period with at least one successful sexual intercourse attempt (68. The proportions of patients with penile pain among those allocated to various alprostadil/prazosin combinations were: 23. The corresponding proportions of patients with urethral pain with respect to various alprostadil/prazosin combinations were: 6.
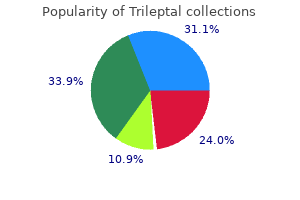
A patient considered to have severe symptoms of anxiety following the further assessment should, where possible, have confirmation of an anxiety disorder diagnosis before any treatment options are initiated. Facilitate a safe environment and one-to-one observation, and initiate appropriate harm-reduction interventions to reduce risk of harm to self and/or others. It is suggested that the clinical team making a patient referral for the treatment of anxiety review with the patient in a shared decision process, the reason(s) for and potential benefits from the referral. For optimal management of moderate to severe or severe anxiety, consider pharmacological and/or non-pharmacological interventions delivered by appropriately trained individuals. Management must be tailored to individual patients, who should be fully informed of their options. For a patient with mild to moderate anxiety, the primary oncology team may choose to manage the concerns by usual supportive care management. The choice of an anxiolytic should be informed by the side effect profiles of the medications, tolerability of treatment, including the potential for interaction with other current medications, response to prior treatment and patient preference. Caution is warranted with respect to the use of benzodiazepines in the treatment of anxiety, specifically over the longer term. These medications carry an increased risk of abuse and dependence and are associated with side effects that include cognitive impairment. As a consequence, use of these medications should be time limited in accordance with established psychiatric guidelines. Offer support and provide education and information about anxiety and its management to all patients and their families and what specific symptoms or symptom worsening warrant a call to the physician or nurse. Use of outcome measures should be routine (minimally pre and post treatment) to a) gauge the efficacy of treatment for the individual patient; b) monitor treatment adherence; and c) evaluate practitioner competence. Longer periods of tapering are often necessary with benzodiazepines, particularly with potent or rapidly eliminated medications. The frequency of visits and testing should consider the data showing that 80% of recurrences occur in the first 2 to 2. Patients at a higher risk of recurrence should be considered for testing in the more frequent end of the range. For high-risk patients, it is reasonable to consider imaging every 6 to 12 months for the first 3 years. Clinician judgment, considering risk status, should be used to determine the frequency of pelvic scans (eg, annually for 3 to 5 years). For those patients who have not received pelvic radiation, a rectosigmoidoscopy should be performed every 6 months for 2 to 5 years. A surveillance colonoscopy should be performed approximately 1 year after the initial surgery. The frequency of subsequent surveillance colonoscopies should be dictated by the findings of the previous one, but they generally should be performed every 5 years if the findings of the previous one are normal. If a complete colonoscopy was not performed before diagnosis, a colonoscopy should be done as soon as reasonable after completion of adjuvant therapy and not necessarily at the 1-year time point. If a patient is not a surgical candidate or a candidate for systemic therapy because of severe comorbid conditions, surveillance tests should not be performed. Recommendation Any new and persistent or worsening symptoms warrant the consideration of a recurrence. What can health care providers do to educate patients about the possibility of reduced fertility resulting from cancer treatments and to introduce them to methods to preserve fertility? Health care providers caring for adult and pediatric patients with cancer (including medical oncologists, radiation oncologists, gynecologic oncologists, urologists, hematologists, pediatric oncologists, surgeons, and others) should address the possibility of infertility as early as possible before treatment starts. Another discussion and/or referral may be necessary when the patient returns for follow-up and if pregnancy is being considered. What is the quality of evidence supporting current and forthcoming options for preservation of fertility in males? Although sperm counts and quality of sperm may be diminished even before initiation of therapy, and even if there may be a need to initiate chemotherapy quickly such that there may be limited time to obtain optimal numbers of ejaculate specimens, these concerns should not dissuade patients from banking sperm. Intracytoplasmic sperm injection allows the future use of a very limited amount of sperm; thus, even in these compromised scenarios, fertility may still be preserved.
Also includes information on clinical trials, reimbursement assistance programs, and a caregiver education course. Their website has a special section for patient information and educational resources. The program also helps find alternative services and resources, including those relating to clothing, transportation, groceries, hospice, and other financial needs. Information is available in English, Spanish, Chinese, Russian, Tagalog, Korean, and Vietnamese. Glossary of Common Cancer Terms In addition to this list, you may want to use the online glossary found on the National Cancer Institute website at Adjuvant chemotherapy: One or more anti-cancer drugs used in combination with surgery or radiation therapy as a part of the treatment of cancer. Aspiration: the process of removing fluid or tissue, or both, from a specific area. Benign tumor: A swelling or growth that is not cancerous, does not spread from one part of the body to another, and is usually not life-threatening. A biopsy is a common way of determining if a person has cancer and, if so, what type it is. They are produced in bone marrow and consist of (1) red cells (which bring oxygen to tissues and take carbon dioxide from them), (2) white blood cells (which fight invading germs, infections, and allergy-causing agents), and (3) platelets (which are responsible for clotting). Blood counts indicate the number of blood cells (red cells, white cells, and platelets) circulating in your bloodstream. The replacement marrow may be taken from the patient before treatment or may be donated by another person. Cancer: A general term for more than 100 diseases characterized by abnormal and uncontrolled growth of cells. Cancer fatigue: A certain type of fatigue associated with the cancer experience that has physical, social, and psychological impact. Case manager: A person hired by your insurance company or hospital to evaluate your ongoing care. Clinical trial: Testing on a group of human subjects that utilizes existing, new, or experimental treatments for a particular disease. Computed tomography: Computer-generated cross-sectional images of a portion of the body. Durable power of attorney: A legal document that lets you appoint someone to make health decisions for you if you become unable to do so for yourself. It should include specific information about the timing and content of recommended follow-up (for example, screening tests for recurrences and/or secondary cancers, follow-up visits with the oncologist, etc. Grade (grading): A system used to categorize how quickly a tumor is likely to grow and spread. The grade of a tumor depends on how abnormal the cancer cells look under a microscope. Healthcare proxy: A person you appoint as your agent to make healthcare decisions for you if you become unable to do so for yourself. Hematologic cancer: A cancer affecting blood-forming cells in the bone marrow, such as leukemia and multiple myeloma. Hormone therapy: Treatment that prevents certain cells from getting the hormones they need to grow, such as treating cancer cells to keep them from receiving hormones, or treating the glands that produce hormones, or surgery to remove glands that produce the hormones, such as the ovaries that produce estrogen, or the testicles that produce testosterone. Hospice: Care for the terminally ill and supportive services for patients and their families. Hyperalimentation: the intravenous administration of a highly nutritious solution. Immune system: the complex group of cells and organs that defend the body against infection and disease. Immunotherapy: Use of the immune system or the products of the immune system to control, damage, or destroy malignant cells (see Biological therapy). Immunosuppression: Weakening of the immune system, causing a lowered ability to fight infection and disease. Informed consent: the legal standard that states that a patient must know certain risks and benefits before agreeing to undergo therapy. It stimulates the growth of certain disease-fighting blood cells in the immune system.
Additional information: