"Discount finasteride 1 mg fast delivery, hair loss cure 2012".
Y. Josh, M.A., M.D., M.P.H.
Deputy Director, Rush Medical College
Please Consider the Environment Before Printing this Document Table of Contents Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure. Any information we determine not to disclose may ultimately be deemed significant by you or others with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. If the top-line data that we report differ from final results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, product candidates may be harmed, which could seriously harm our business. We may not be successful in our efforts to continue to create a pipeline of product candidates or to develop commercially successful products. If we fail to successfully identify and develop additional product candidates, our commercial opportunity may be limited. One of our strategies is to identify and pursue clinical development of additional product candidates through our Therapeutic Vector Evolution platform technology. Our Therapeutic Vector Evolution platform technology may not produce a pipeline of viable product candidates, or our competitors may develop platform technologies that render our Therapeutic Vector Evolution platform technology obsolete or less attractive. Our research methodology may be unsuccessful in identifying potential product candidates or our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make them unmarketable or unlikely to receive marketing approval. Identifying, developing and obtaining regulatory approval and commercializing additional product candidates will require substantial additional funding beyond the net proceeds of this offering and is prone to the risks of failure inherent in drug development. If we are unable to successfully identify, acquire, develop and commercialize additional product candidates, our commercial opportunity may be limited. We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than us. We may face competition with respect to any product candidates that we seek to develop or commercialize in the future from major pharmaceutical companies and biotechnology companies worldwide. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing, and commercialization. Certain of our competitors have commercially approved products for the treatment of the diseases that we are pursuing or may pursue in the future, including Roche, Sanofi, Takeda and Vertex. These drugs are well established therapies and are widely accepted by physicians, patients and third-party payors, which may make it difficult to convince these parties to switch to our product candidates. Companies that we are aware are developing therapeutics in the lysosomal storage diseases, lung diseases, muscular dystrophies and ophthalmological diseases areas include large companies with significant financial resources, such as Allergan, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and Vertex, and biopharmaceutical companies such as Avrobio, Freeline, Sangamo and Sarepta. Please Consider the Environment Before Printing this Document Table of Contents ophthalmologic indications, any products we may develop may also face competition from other types of therapies, such as gene-editing therapies and drug delivery devices. Many of our current or potential competitors, either alone or with their strategic partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to , or necessary for, our product candidates. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any products that we may develop. Furthermore, currently approved products could be discovered to have application for treatment of lysosomal storage, lung, muscular dystrophy and ophthalmologic indications, which could give such products significant regulatory and market timing advantages over any of our product candidates. Additionally, products or technologies developed by our competitors may render our potential product candidates uneconomical or obsolete, and we may not be successful in marketing any product candidates we may develop against competitors. If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any product candidates we may develop, we may not be successful in commercializing those product candidates if and when they are approved. We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource these functions to third parties.
In i t i a l l y the c a r d i o g e n i c a r e a a n d the p e r i c a r d i a l c a v i t y a r e i n f r o n t o f the b u c c o p h a r y n g e a l me mb r a n d a y sB. S c a n n i n g e l e c t r o n mi c r o g r a p h o f a mo u s e e mb r y o a t a. D u r i n g the s e e v e n t s, the my o c a r d i u m t h i c k e n s a n d s e c r e t e s a t h i c k l a y e r o f e xt r a c e l l u l a r ma t r i x, r i c h i n h y a l u r o n i c a c i d, t h a t s e p a r a t e s i t f r o m the e n d o the l i u m (F i g s. In a d d i t i o n, me s o the l i a l c e l l s o n the s u r f a c e o f the s e p t u m a) t r a n s v e r s u m f o r m the e p i c a r d i u me a r the s i n u s v e n o u s a n d mi g r a t e o v e r the pro n h e a r t t o f o r m mo s t o f etp ie a r d i u mT h e r e ma i n d e r o f the e p i c a r d i u m i s d e r i v e d hc. T h u s, the h e a r t t u b e c o n s i s t s o f t h r e e l a y e r)s t: h(ee n d o c a r d i u,mf o r mi n g the i n t e r n a l e n d o the l i a l a l i n i n g o f the h e abt; t (em y o c a r d i u mf o r mi n g the mu s c u l a r w a l l;c)a nh e(r) h, td e p i c a r d i u m r v i s c e r a l p e r i c a r d i, uc o v e r i n g the o u t s i d e o f the t u b. T r a n s v e r s e s e c t i o n s t h r o u g h e mb r y o s a t d i f f e r e n t s t a g e s o f 3 d e v e l o p me n t, s h o w i n g f o r ma t i o n o f a s i n g l e h e a r t t u b e f r o m p a i r e d p r i mo r d i a. F u s i o n o c c u r s o n l y i n the c a u d a l r e g i o n o f the h o r s e s h o e - s h a p e d t u b e (si e e 1 2. F r o n t a l v i e w o f a n e mb r y o s h o w i n g the h e a r t i n the p e r i c a r d i a l 4 cavity and the developing gut tube with the anterior and posterior intestinal p o r t a l s. T h e o r i g i n a l p a i r e d t u b e s o f the h e a r t p r i mo r d i a l h a v e f u s e d i n t o a single tube. The caudal pole of the heart tube, including the sinus venosus, is e mb e d d e d i n the s e p t u m t r a n s v e r s u m, w h i l e the o u t f l o w t r a c t l e a d s t o the aortic sac and aortic arches. Form ation of the Cardiac Loop The heart tube continues to elongate and bend on day 23. The cephalic portion of the t u b e b e n d s v e n t r a l l y, c a u d a l l y, a n d t o tF i. W h i l e the c a r d i a c l o o p i s f o r mi n g, l o c a l e xp a n s i o n s b e c o me v i s i b l e t h r o u g h o u t the l e n g t h o f the ta tb ea l Tp o r t i o nn i t i a l l y a u ri. T h i s p o r t i o n wti r la bo rc u lt a t e d p a r t o f l f e m he the r i g h t v e n t r i c l eg s. T h e mi d p o r t i o n, c h e u s c o r d,i s i l l (F i a) t on w form the outflow tracts of both ventricles. The distal part of the bulbus, the t r u n c u s a r t e r i o s u si l l f o r m the r o o t s a n d p r o xi ma l p o r t i o n o f the a o r t a a n d, w p u l mo n a r y a r t e rF i g. E v i d e n c e s u g g e s t s t h a t o r g a n i za t i o n o f the s e F A s e g me n t s i s r e g u l a t e d b y h o me o b o x g e n e s i n a ma n n e r s i mi l a r t o t h a t f o r the c r a n i o c a u d a l a xi s o f the e mb r y o h s e ee r)6 C (apt. T h e d e v e l o p i n g 5 e n d o c a r d i a l h e a r t t u b e a n d i t s i n v e s t i n g l a y e r b u l g e i n t o the p e r i c a r d i a l c a v i t y. F r o n t a l v i e w o f the h e a r t t u b e u n d e r g o i n g l o o p i n g i n the p e r i c a r d i a l c a v i t y. T h e p r i mi t i v e v e n t r i c l e i s mo v i n g v e n t r a l l y a n d t o the r i g h t, w h i l e the a t r i a l r e g i o n i s mo v i n g d o r s a l l y a n d t o t(h e r lows) ar e f t. At the e n d o f l o o p f o r ma t i o n, the s mo o t h - w a l l e d h e a r t t u b e b e g i n s t o f o r m p r i mi t i v e t r a b e c u l a e i n t w o s h a r p l y d e f i n e d a r e a s j u s t p r o xi ma l a n d d i s t a l t o the p r i ma r y i n t e r v e n t r i c u l a r f o r a me g. T h e p r i mi t i v e v e n t r i c l e, w h i c h i s n o w t r a b e c u l a t e d, i s r ia l lie dv teh ee f t p c m ti l v e n t r i c l eL i k e w i s e, the t r a b e c u l a t e d p r o xi ma l t h i r d o f the b u l b u s c o r d i s ma y b. The conotruncal portion of the heart tube, initially on the right side of the p e r i c a r d i a l c a v i t y, s h i f t s g r a d u a l l y t o a mo r e me d i a l p o s i t i o n. T h i s c h a n g e i n p o s i t i o n i s the r e s u l t o f f o r ma t i o n o f t w o t r a n s v e r s e d i l a t i o n s o f the a t r i u m, b u l g i n g o n e a c h s i d e o f the b u l b u s c o ri d iss (2. D e xt r o c a r d i a ma y c o i n c i d eSwti uh i n v e r s,u s c o mp l e t e r e v e r s a l o f i ts a a s y mme t r y i n a l l o r g a n s. S i t u s i n v e r s u s, w h i c h o c c u r s i n 1 / 7, 0 0 0 i n d i v i d u a l s, u s u a l l y i s a s s o c i a t e d w i t h n o r ma l p h y s i o l o g y, a l t h o u g h the r e i s a s l i g h t r i s k o f h e a r t d e f e c t s. In o the r c a s e s, s i d e d n e s s i s r a n d o m, s u c h t h a t s o me o r g a n s a r e r e v e r s e d a n d o the r s a r e n o t; th e s eir o t a x y. T h e s p l e e n r e f l e c t s the differences: those with left-sided bilaterality have polysplenia; those with right-sided bilaterality have asplenia or hypoplastic spleen. Patients with l a t e r a l i t y s e q u e n c e s a l s o h a v e i n c r e a s e d i n c i d e n c e s o f o the r ma l f o r ma t i o n s, e s p e c i a l l y h e a r t d e f e c t s. G e n e s r e g u l a t i n g s i d e d n e s s a r e e xp r e s s e d d u r i n g g a s t r u l a t i o n (s C e a p t e r)5 eh. T h e b u l b u s c o r d i s i s d i v i d e d i n t o the t r u n c u s a r t e r i o s u s, c o n u s c o r d i s, a n d t r a b e c u l a t e d p a r t o f the r i g h t v e n t renl e i. Br ok i c l, C S c a n n i n g e l e c t r o n mi c r o g r a p h o f the h e a r t o f a s i mi l a r - s t a g e d h u ma n e mb r y o s h o w i n g a v i e w s i mi l aB.
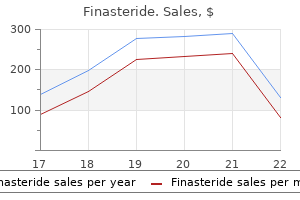
In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Purchasers of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse. Restricted stock units may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions continuing service with us or our affiliates, the attainment of performance goals and/or such other conditions as the plan administrator may determine. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. The plan administrator has broad discretion to equitably adjust the provisions of the 2015 Plan, as well as the terms and conditions of existing and future awards, to prevent the diminution or enlargement of intended benefits and facilitate necessary or desirable changes in the event of certain transactions and events affecting our common stock, such as a dividend or other distribution (whether in the form of cash, Shares, other securities or other property), recapitalization, reincorporation, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, reclassification, repurchase or exchange of our shares or our other securities, or other change in our corporate structure. In the event of a merger with or into another corporation or other entity or a change in control (as defined in the 2015 Plan), the plan administrator may provide, in any combination hereof, subject to the applicable award agreement and without the participants consent, that (i) the surviving entity assume outstanding awards or substitute economically equivalent awards for such outstanding awards; (ii) that the participants awards will terminate upon or immediately prior to the consummation of the merger or change in control; (iii) outstanding awards will vest and become exercisable, realizable or payable, or restrictions applicable to an award will lapse, in whole or in part prior to or upon consummation of such merger or change in control; and (iv) the termination of an award in exchange for any combination of cash, property or other rights, if any, selected by the administrator in its sole discretion, equal in value to the cash or other property that would have been attained upon the exercise of such award or realization of the participants rights as of the date of the occurrence of the transaction. The administrator may also make appropriate adjustments to awards under the 2015 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. The plan administrator may establish sub-plans under the 2015 Plan, subject to the share limits described above, containing such limitations and other terms and conditions that the plan administrator determines is necessary or desirable to satisfy blue sky, securities, tax or other laws of various jurisdictions in which we intend to grant awards or qualifying for favorable tax treatment under applicable foreign laws. With limited exceptions for gifts or executors of the participants estate upon the participants death, in connection with certain acquisitions or a change in control, and transfers to us, awards under the 2015 Plan are generally non-transferable prior to exercise or delivery and are exercisable only by the participant. Please Consider the Environment Before Printing this Document Table of Contents check, promissory note, shares of our common stock that meet specified conditions, such other consideration and method of payment for the issuance of shares, to the extent permitted by applicable laws, by net exercise, a market sell order or any combination thereof. Our board of directors may amend or terminate the 2015 Plan at any time; however, (i) no amendment or termination may adversely affect an outstanding award without the affected participants written consent and (ii) except in connection with certain changes in our capital structure, stockholder approval will be required for (A) any amendment that increases the number of shares available under the 2015 Plan or extends the term of the 2015 Plan, or (B) as required under applicable law. No award may be granted pursuant to the 2015 Plan after the ten year anniversary of the date the 2015 Plan, as amended or restated, was approved by our board of directors or our stockholders (whichever was earlier), however, we will cease granting awards under the 2015 Plan upon effectiveness of the 2019 Plan. Please Consider the Environment Before Printing this Document Table of Contents than 15% of their compensation. Such payroll deductions may be expressed as either a whole number percentage or a fixed dollar amount, and the accumulated deductions will be applied to the purchase of shares on each purchase date. However, a participant may not purchase more than 60,000 shares in each offering period and may not subscribe for more than $25,000 in fair market value of shares of our common stock (determined at the time the option is granted) during any calendar year. The option purchase price will be the lower of 85% of the closing trading price per share of our common stock on the trading date immediately preceding the first day of an offering period in which a participant is enrolled or 85% of the closing trading price per share on the trading day immediately preceding the last day of each purchase period. Upon exercise, the participant will purchase the number of whole shares that his or her accumulated payroll deductions will buy at the option purchase price, subject to the participation limitations listed above. A participant may cancel his or her payroll deduction authorization at any time prior to the end of the offering period. Upon cancellation, the participant will have the option to either (i) receive a refund of the participants account balance in cash without interest or (ii) exercise the participants option for the current offering period for the maximum number of shares of common stock on the applicable purchase date, with the remaining account balance refunded in cash without interest. Following at least one payroll deduction, a participant may also decrease (but not increase) his or her payroll deduction authorization once during any offering period. If a participant wants to increase or decrease the rate of payroll withholding, he or she may do so effective for the next offering period by submitting a new form before the offering period for which such change is to be effective. Any such attempt at assignment, transfer, pledge or other disposition will not be given effect. Adjustments upon changes in recapitalization, dissolution, liquidation, merger or asset sale. Please Consider the Environment Before Printing this Document Table of Contents purchase date to take place before the date of our dissolution or liquidation. If we undergo a merger with or into another corporation or sell all or substantially all of our assets, each outstanding option will be assumed or an equivalent option substituted by the successor corporation or the parent or subsidiary of the successor corporation. If the successor corporation refuses to assume the outstanding options or substitute equivalent options, then any offering period and purchase period then in progress will be shortened by setting a new purchase date to take place before the date of our proposed sale or merger. We will notify each participant of such change in writing at least ten business days prior to the new exercise date. Sales and Purchases of Securities Series B Convertible Preferred Stock Financing In August 2018, we issued an aggregate of 5,154,632 shares of our Series B convertible preferred stock at $17. The table below sets forth the aggregate number of shares of Series B convertible preferred stock sold to our directors, executive officers or owners of more than 5% of a class of our capital stock, or an affiliate or immediate family member thereof: Number of Shares of Series B Convertible Preferred Stock Aggregate Purchase Price ($) Name Pfizer Inc. Employment Agreements We have entered into employment agreements with our executive officers.
C e l l a h 5g t d e a t h i n the i n t e r d i g i t a l s p a c e s p r o d u c e s s e p a r a t i o n oC. L o w e r e xt r e mi t y o f a n e a r l y 6 - w e e k e mb r y o, i l l u s t r a t i n g the f i r s t 5 h y a l i n e c a r t i l a g e mo dB,l s. C o mp l e t e s e t o f c a r t i l a g e mo d e l s a t the e n d o f e C the s i xt h w e e k a n d the b e g i n n i n g o f the e i g h t h w e e k, r e s p e c t i v e l y. Chondrocytes toward the shaft side (diaphysis) undergo hypertrophy and a p o p t o s i s a s the y mi n e r a l i ze the s u r r o u n d i n g ma t r i x. O s t e o b l a s t s b i n d t o the mi n e r a l i ze d ma t r i x a n d d e p o s i t b o n e ma t r i c e s. L a t e r, a s b l o o d v e s s e l s i n v a d e the e p i p h y s e s, s e c o n d a r y o s s i f i c a t i o n c e n t e r s f o r m. G r o w t h o f the b o n e s i s ma i n t a i n e d b y p r o l i f e r a t i o n o f c h o n d r o c y t e s i n the g r oF i tgh p l. M o l e c u l a r R e g u l a t i o n o f L i m b D e ve l o p m e n t P o s i t i o n i n g o f the l i mb s a l o n g the c r a n i o c a u d a l a xi s i n the f l a n k r e g i o n s o f the e mb r y o i s r e g u l a t e d b yHtO e g e n e se xp r e s s e d a l o n g t h i s a xi s. T h e s e hX h o m e o b o x e n e s a r e e xp r e s s e d i n o v e r l a p p i n g p a t t e r n s f r o m h e a d t o t a i l (s e e g C h a p t e r)6 w i t h s o me h a v i n g mo r e c r a n i a l l i mi t s t h a n o the r s. O n c e p o s i t i o n i n g a l o n g the c r a n i o c a u d a l a xi s i s d e t e r mi n e d, g r o w t h mu s t b e r e g u l a t e d a l o n g the p r o xi mo d i s t a l, a n t e r o p o s t e r i o r, a n d d o r s o v e n Fria l. T h i s g e n e i n d u c e s eS ErR 2 s i o n o f xp e s a, h o mo l o g u e o f os ophi l a s er r,at e the b o r d e r b e t w e e n c e l l s t h a t a r e e xp r e s s i n g Dr at Radi c al f r i ngen d t h o s e t h a t a r e n o t. F o r ma t i o n o f the b o r d e r i t s e l f i s a s s i s t e d b y eE n ge s s li e d - o f xp r r a i o n 1 i n v e n t r a l e c t o d e r m c e l l s, s i n c e t h i s g e n e r e p r e s s e s e xp r e s c al nf ronge Radi s i o i f. Af t e r the r i d g e i s e s t a b l i s h e d, i t e xp rG F s e s dF G F 8 w h i c h ma i n t a i n the F es4an, p r o g r e s s z o n e h e r a p i d l y p r o l i f e r a t i n g p o p u l a t i o n o f me s e n c h y me c e l l s a d j a c e n t, t t o the r i d g e i (g. As g r o w t h o c c u r s, me s e n c h y ma c e l l s a t the p r o xi ma l e n d o f the p r o g r e s s zo n e b e c o me f a r the r a w a y f r o m the r i d g e and its influence and begin to slow their division rates and to differentiate. T h u s, f o r e xa mp l e, d i g i t s a p p e a r i n the p r o p e r o r d e r, w i t h the t h u mb o n the r a d i a l (a n t e r i o r) s i d. T h e s e t w o g e n e s a r e a l s o i n t i ma t e l y l i n k e d i n s i g n a l i n g p a t h w a y s i n Dr os ophi l, aa n d t h i s i n t e r a c t i o n i s c o n s e r v e d i n v e r t e b r a t e s. In f a c t, a l l o f the p a t t e r n i n g g e n e s i n the l i mb h a v e f e e d b a c k l o o p s. Al t h o u g h p a t t e r n i n g g e n e s f o r the l i mb a xe s h a v e b e e n d e t e r miH Od, i t i s the ne X g e n e st h a t r e g u l a t e the t y p e s a n d s h a p e s o f the b o n e s oF i tg. Af t e r the r i d g e i s e s t a b l i s h e d, i t e xp r e s s e s F G F 4 a n d F G F 8 t o ma i n t a i n the p r o g r e s s zo n e, the r a p i d l y p r o l i f e r a t i n g me s e n c h y me c e l l s a d j a c e n t t o the r i dB. Clinical Corre late s Bone Age R a d i o l o g i s t s u s e the a p p e a r a n c e o f v a r i o u s o s s i f i c a t i o n c e n t e r s t o d e t e r mi n e w h e the r a c h i l d h a s r e a c h e d h i s o r h e r p r o p e r ma t u r a t i o n a g. U s e f u l i n f o r ma t i o n a b o uo n e a g e s o b t a i n e d f r o m o s s i f i c a t i o n s t u d i e s i n the h a n d s b t i and wrists of children. Prenatal analysis of fetal bones by ultrasonography p r o v i d e s i n f o r ma t i o n a b o u t f e t a l g r o w t h a n d g e s t a t i o n a l a g. Lim b De fe cts L i mb ma l f o r ma t i o n s o c c u r i n a p p r o xi ma t e l y 6 p e r 1 0, 0 0 0 l i v e b i r t h s, w i t h 3. T h e s e defects are often associated with other birth defects involving the craniofacial, c a r d i a c, a n d g e n i t o u r i n a r y s y s t e ms. S o me t i me s the l o n g b o n e s a r e a b s e n t, a n d r u d i me n t a r y h a n d s a n d f e e t a r e a t t a c h e d t o the t r u n k b y s ma l l, i r r e g u l a r l y s h a p e d b o n e s h o c o m e l i, aa f o r m o f me r o me l i a) (s e e 9. S o me t i me s a l l s e g me n t s o f the e xt r e mi t i e s a r e p r e s e n t b u t a b n o r ma l l y s h o r t (m i c r o m e l i. F o r e xa mp l e, ma n y c h i l d r e n w i t h l i mb ma l f o r ma t i o n s w e r e b o r n b e t w e e n 1 9 5 7 a n d 1 9 6 2. M a n y mo the r s o f the s e i n f a n t s h a d t a k teh a l i d o m i d ea d r u g w i d e l y u s e d a s a s l e e p i n g p i l l a n d n, a n t i n a u s e a n t. It w a s s u b s e q u e n t l y e s t a b l i s h e d t h a t t h a l i d o mi d e c a u s e s a c h a r a c t e r i s t i c s y n d r o me o f ma l f o r ma t i o n s c o n s i s t i n g o f a b s e n c e o r g r o s s d e f o r mi t i e s o f the l o n g b o n e s, i n t e s t i n a l a t r e s i a, a n d c a r d i a c a n o ma l i e s. S t u d i e s i n d i c a t e t h a t the mo s t s e n s i t i v e p e r i o d f o r t e r a t o g e n - i n d u c e d l i mb ma l f o r ma oi u r ts i s the f t on h a n d f i f t h w e e k s d e v e l o p me n t. S o me t i me s the d i g i t s a r e s h o r t e n e db (a c h y d a c t y;l y e eF i g. In 1 p e r 2, 0 0 0 b i r t h s t h i s p r o c e s s f a i l s, a n d the r e s u l t i s f u s i o n b e t w e e n t w o o r mo r e d i g i t s.