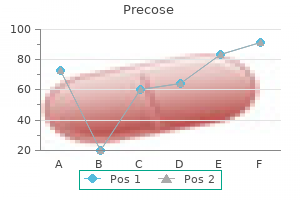
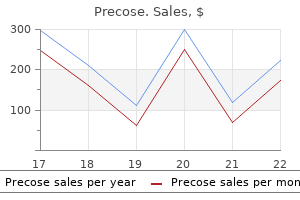
"Purchase precose cheap, diabetes insipidus gestational".
Z. Nemrok, M.B. B.CH. B.A.O., M.B.B.Ch., Ph.D.
Clinical Director, Indiana Wesleyan University
Also, the absence of sedation leads to a low satisfaction experience for patients, such that they are less willing to repeat the examination compared with colonoscopy. However, endoscopic screening in general is more effective in the left than the right side of the colon, and there is no clear reason why flexible sigmoidoscopy should not be recommended at 10-year intervals, similar to the recommendation for colonoscopy. Advantages of capsule colonoscopy are the achievement of endoscopic imaging without an invasive procedure and avoiding the risks of colonoscopy. Disadvantages are that the bowel preparation is more extensive than that for colonoscopy. Also, because the logistics of performing same-day colonoscopy on patients with positive capsule studies are quite difficult, most patients with positive studies will require repreparation and colonoscopy on a separate day. In a large screening trial in 884 patients, capsule colonoscopy had 88% sensitivity for detecting patients with a conventional adenoma 6 mm in size but was ineffective for the detection of serrated lesions, and 9% of patients had technically failed examinations for inadequate cleansing or rapid transit of the capsule. Numerous modeling studies have addressed the relative cost-effectiveness of 2 or more screening tests. The conclusions of the models frequently vary, likely depending in part on the assumptions of the respective models. Some models support the cost-effectiveness of risk-stratified approaches to screening. The advantage of the Septin9 test is that it is a serum assay and is at least potentially more convenient for patients. The uncertainties regarding the true clinical utility of Septin 9 makes shared decision-making difficult. Unlike primary care physicians, the main role of gastroenterologists in the screening process is to perform colonoscopy on patients referred for primary colonoscopy screening or for colonoscopy to evaluate other positive screening tests. As such, a primary task of gastroenterologists is to perform high-quality colonoscopy and costeffective follow-up. Two large studies have validated the adenoma detection rate as a predictor of cancer prevention by colonoscopy. Patients should expect a prospective colonoscopist to provide his or her adenoma detection rate, which should meet or exceed recommended minimum thresholds (Table 3). When compared using simulation models that are dependent on assumptions about natural history of disease, patient acceptance of screening, and test performance, several tests appear to be similarly effective. On the other hand, the core concept underlying sequential testing is that offering the "best" test(s) first optimizes the sensitivity and/or cost-effectiveness of screening and still leaves the opportunity to offer other tests when patients decline. Risk stratification may also take into account factors such as cigarette smoking, diabetes, and obesity. The rationale for placing tests in the tier 2 and 3 categories follows from the discussion above of individual tests. For example, flexible sigmoidoscopy screening has strong evidence to support its use. However, offering capsule colonoscopy as a tier 2 test in a sequential methodology currently would often lead to frustration because reimbursement for screening capsule colonoscopy is seldom available at this time, and the test itself is frequently not available. The onerous bowel preparation and the lack of systems to accomplish same-day colonoscopy in most patients with a positive capsule colonoscopy are additional factors placing capsule colonoscopy in the tier 3 category at this time. Predominant reliance on tier 1 tests offers modalities with optimal effectiveness, costeffectiveness, complete colon screening, proven popularity with patients, and a simplified discussion (compared with offering 5-7 different tests) and still leaves room to offer other tests in a sequential fashion. We recommend that physicians performing screening colonoscopy measure quality, including the adenoma detection rate (strong recommendation, high-quality evidence). We no longer recommend that persons with a family history of adenomas in a first-degree relative undergo early screening, unless there is clear documentation of an advanced adenoma in a first-degree relative. If a colonoscopy and/or pathology report(s) is available for a family member that documents an advanced adenoma or there is a report of a polyp requiring surgical resection, an advanced adenoma in a family member is considered established. These considerations regarding adequate documentation of advanced precancerous neoplasms in first-degree relatives before intensifying screening apply to documentation of both advanced adenomas and advanced serrated lesions. The yield of colonoscopic screening in firstdegree relatives of persons with advanced adenomas is substantially increased. Persons with bleeding symptoms evaluated with tests other than colonoscopy (eg, sigmoidoscopy) should have a bleeding source identified and treated, and the patient should be followed to resolution of the symptom. Additional study of the benefits and harms of screening in persons <50 years is warranted, perhaps particularly in persons with known colorectal risk factors such as cigarette smoking, diabetes mellitus, and obesity. There are few data on the yield of screening in African Americans before age 50 or whether earlier screening improves outcomes.
Stoma size (diameter), shape (oval, round, or irregular), and structure (flush, spout, mushroom, pro- lapse) as well as viability of stoma(s). Presence of lacerations (may look like white line near base of stoma) or other lesions. Integrity of the mucocutaneous junction (area where stoma mucosa borders peristomal skin). Sutures may be visualized at the junction if stoma is new within past few days to two to three weeks. Peristomal skin condition and contours including creases, depressions, irritation or superficial skin loss (denudement), presence of rash, character of peristoma wounds (see Table 4). Pouch change date and time, reason for change, and location of leakage/tunneling of effluent, if present. Cut-to-fit pouches are generally used on children because of the variations in stoma size postoperatively and with normal physical growth. This requires measuring and cutting an opening on each pouch barrier to fit the stoma(s). Generally, multiple intestinal stomas may be pouched within the same appliance, with exceptions (see "Pouch Selection and Ostomy Care, Ostomy Care, Education. Urinary and intestinal stomas should always be pouched separately, except at the discretion of the surgeon. Stomas decrease in size over six to eight weeks postoperatively and must be re-measured with each pouch change until their size has stabilized. Stomas created to correct or alleviate conditions in which the bowel is dilated. Stomas should be re-measured weekly in a growing child, and whenever a previously adequate pouching system begins to fail. A stomameasuring device (included in each box of pouches) should be used to measure stoma size at the level of the mucocutaneous junction to determine the size aperture to cut in the pouch wafer. The aperture for the stoma should be no more than 1/8-inch in diameter larger than the stoma diameter at skin level to minimize skin exposure to effluent, and no less than equal to the stoma diameter to prevent trauma to the stoma or obstruction of the stoma opening. The opening for the stoma can be centered on the wafer, or away from center if the pouch would benefit from being shifted from landmarks on the abdomen. If the wafer has a pre-cut starter hole, the tracing for the stoma opening must encompass the entire starter hole to prevent excess skin exposure to effluent. Pre-cut starter holes limits the ability to shift the pouch away from abdominal landmarks. If using an appliance with a starter hole, and the hole is not in the best location for the stoma, it can be covered from inside the pouch, or on top of the wafer if a two-piece system, with a piece of hydrocolloid dressing or the remnant left from cutting the new stoma opening. Irregularly shaped stomas, grouping of stomas, or stoma(s) in close proximity to abdominal landmarks may require a custom template or tracing. Place a sheet of transparent, flexible plastic or cellulose over the abdomen, and trace the stoma base and other landmarks onto the plastic with a felt-tip marker. This pattern can be cut and then traced onto a pouch wafer, taking care not to reverse the pattern. If the pouch will be worn angled to the side rather than toward the feet and the stoma shape is not round, the template should be angled on the pouch to mirror the relationship of the pouch to the stoma/abdomen prior to tracing the stoma opening on the paper backing of the wafer. Before cutting the stoma aperture, pull the pouch front away from the wafer, creating a pocket of air between the wafer and the pouch front. Before removing the paper backing from the pouch wafer, place the wafer over the stoma(s) to deter- mine that the stoma opening is a correct fit. Also assess if any of the wafer will need to be trimmed away to accommodate groin movement, the moist umbilicus of a newborn, or any medical devices that may be in close proximity to the ostomy. Trim the opening as needed, refitting the wafer over the stoma(s) with each correction, until the opening is the correct size and shape to fit the stoma(s). Take care not to cut away too much of the wafer with each correction, or the opening may become too large, exposing peristomal skin, and the pouch will not be useable.
The luminal surface (center of the 40x view of colonic mucosa slide) is smooth and consists of pale cells (called Goblet cells), absorptive cells, and enteroendocrine cells that make up the mucosa. The free surface of the cell, facing a lumen, is referred to as the cell apex and the opposite surface is the cell base. The lateral borders should be seen and contain structures that connect the cells together. Note the intense reaction at the apical surface of the epithelial cells and within scattered goblet cells (containing mucin) at the luminal surface. Note the basophilia in the basal compartment and the acidophilia in the apical (luminal) compartment of the cytoplasm. These cells contain secretory granules, which release digestive enzymes into the lumen of the acinus. The lumens of the acini converge into 40x view of interlobular ducts, gallbladder mucosa eventually merging to lumen secretory granules #115 Gall bladder H&E - Microvilli Examine the cells lining the lumen of this organ, the gall bladder. A border may be identified at the apex of the cells, which has slightly different optical properties from the remainder of the cell. Under optimum conditions faint striations, oriented parallel to the long axis of the cell, are seen in the border. These are difficult to resolve at the light microscopic level, but with electron microscopy, these striations are seen to be precisely arranged microvilli, containing cores of actin filaments. The lining cells of the small intestine will be studied in more detail at a later time. At the apex of these cells note the pink line, which indicates the presence of the basal bodies that give rise to the cilia. Consult electron micrographs for the content and morphology of cilia and their basal bodies. During prophase, the nuclear envelope disperses, replicated chromosomes condense, and the two sister chromatids become attached at a site called the centromere. At anaphase B, the sister chromatids continue to migrate toward the poles and the microtubules of the spindle elongate. During telophase, the sister chromatids reach the poles, the nuclear envelope re-forms and the chromosomes decondense. Cytoplasmic division usually begins in anaphase and is complete by the end of telophase. Slide #112: Whitefish Mitosis the whitefish embryo has been stained with hematoxylin and eosin (H&E). There are examples of cells at all stanges of the cell cycle since the cells are dividing asynchronously. Assess nuclear envelope breakdown, chromosome condensation, mitotic spindle development, and location of condensed chromosomes in the whitefish mitotic cells. On the basis of these parameters, identify and determine the distinguishing features of cells in prophase, metaphase, anaphase (A and B) and telophase. Know the structural characteristics and functional significance of the following organelles and inclusions: nucleus, nucleolus, ribosomes, endoplasmic reticulum (two types), mitochondria, Golgi apparatus, lysosomes, microtubules, cilia, microvilli, glycogen, lipid, peroxisomes. Relate characteristics of particular epithelia to their function, keeping in mind their essential features including junctions, apical modifications, and polarity. An epithelium is a layer or sheet of cells that covers a surface or lines a cavity. Functions of epithelia include formation of a protective layer (epidermis), absorption of water and solutes (intestine), secretion (intestine, various glands) and excretion (kidney tubules). Classification of epithelia is generally based upon two criteria: number of cell layers and cell shape. Simple epithelia are one cell layer thick and stratified epithelia are two or more cell layers thick. Pseudostratified epithelium is an intermediate type that appears stratified but really is one cell layer thick. The shape of epithelial cells may be squamous, cuboidal, or columnar; intermediate forms are often encountered. Stratified epithelia are classified according to the shape of the cells at the free surface and can be squamous, cuboidal, columnar, or transitional. Transitional epithelia line cavities in the urinary tract, which may be distended, and the thickness of the epithelium varies with the degree of distention. Beneath the layer of epithelial cells is an underlying non-cellular structure known as the basal lamina, which is secreted by the epithelial cells.
Syndromes
- Do you find yourself doing purposeless activities (e.g., pacing, hand wringing)?
- Tumors of the face, skin, and other exposed areas
- Defects in your heart valve are causing major heart symptoms, such as chest pain (angina), shortness of breath, fainting spells (syncope), or heart failure.
- Blood loss
- Isopropyl alcohol
- Vomiting
In fully established cases the fissure may be felt as a vertical crack in the anal canal. It includes: - A high fiber diet and high fluid intake with a mild laxative, such as liquid paraffin, to encourage passing of soft, bulky stools - Administration of a local anesthetic ointment or suppository Surgical Measures: Surgical measures are needed when the above measures fail, in chronic fissures with fibrosis, a skin tag or a mucous polyp or recurrent anal fissures. It needs an experienced operator to reduce complications, which include hematoma formation, incontinence and mucosal prolapse. After care: this consists of bowel care, daily bath and softening the stool till wound healing. Since the internal and external (subcutaneous perianal) venous plexus communicate (Porto-systemic anastomosis) engorgement of the internal plexus is likely to lead to involvement of the latter. This arrangement corresponds to the distribution of the superior hemorrhoidal vessels (2 on the right, one on the left) but there can be smaller hemorrhoids in between the three groups. Hemorrhoids are graded based on the degree of prolapse and reducibility in to: First degree hemorrhoids: those confined to the anal canal (do not prolapse out side the anal canal) Second degree hemorrhoids: prolapse on defecation but reduce spontaneously or are replaced manually and stay reduced. Third degree hemorrhoids: prolapse, even apart from defecation, and remain permanently prolapsed outside the anal margin. These give rise to a feeling of heaviness in the rectum - A mucoid discharge frequently accompanies prolapsed hemorrhoids and is due to mucus secretion from the engorged mucus membrane. Anemia-due to persistent/profuse bleeding On examination every patient should undergo at least: Complete abdominal and pelvic examination looking for underlying causes or aggravating factors. Rectal examination: Inspection may show prolapsing hemorrhoids (piles) with or without straining and/or redundant skin folds or skin tags. Unrelieved strangulation/thrombosis may lead to ulceration of the exposed mucus membrane. Management: Any underlying or associated more important condition or disease should be excluded or treated accordingly before commencing specific treatment for hemorrhoids. Hemorrhoids can be managed with: Conservative measures which include: - High fiber-diet for a regular soft and bulky motion Hydrophilic creams or suppositories Local application of analgesic ointment /suppository. This is recommended and usually effective for many patients with early hemorrhoids particularly those secondary to other conditions and likely to regress with removal of the underlying conditions. It appears as an inflamed tense tender and easily visible on inspection of the anal verge. Continuous pain, on the other hand, signifies infection, inflammation or ischemia. Luminal Gallstone Ileus Food bolus Meconium Ileus Malignancy or inflammatory mass Ascaris bolus b. Extra mural Adhesions: Congenital, inflammatory or malignant Hernia(as cause of intestinal obstruction): External or internal hernias Volvulus: small bowel, large bowel etc. As distension increases with time, blood vessels in the bowel will be stretched and narrowed impairing blood flow and leading to ischemia. Absorptive capacity of the gut decreases with a net increase of water and electrolytes secretion into the lumen. There will be increased vomiting which leads to depletion of extra cellular fluid which eventually leads to hypovolemia and dehydration. A strangulated loop dies and perforates to produce severe bacterial peritonitis which is often fatal. Grossly distended abdomen restricts diaphragmatic movement and interferes with respiration. A multiple organ failure will subsequently result if the strangulated loop is not removed. The mesocolic veins then become occluded and the arterial inflow into the twisted loop perpetuates the volvulus until it becomes irreversible. Unless the situation is relieved, perforation may occur due to either pressure necrosis at the base of the twist or to avascular necrosis at the apex. If the deflation fails, laparotomy and derotation of the loop has to be done followed by elective resection to prevent recurrent attacks.