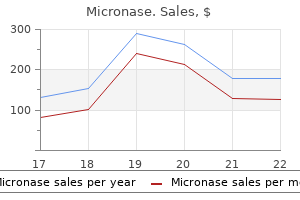
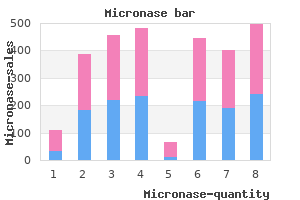
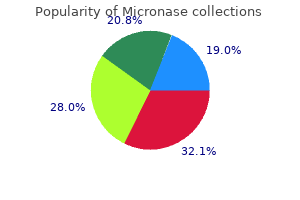
"Buy micronase 2.5 mg online, diabetes symptoms checklist quiz".
A. Urkrass, M.A., M.D., M.P.H.
Clinical Director, The University of Arizona College of Medicine Phoenix
Page 180 of 260 Waiting Period No recommended time frame You should not certify the driver until the treatment has been shown to be adequate/effective, safe, and stable. Recommend not to certify if: As a medical examiner, you believe that the nature and severity of the medical condition and/or the treatment of the driver endangers the safety and health of the driver and the public. You may require the driver to have more frequent physical examinations, if indicated, to adequately monitor driver medical fitness for duty. Other Diseases the fundamental question when deciding if a commercial driver should be certified is whether the driver has a condition that so increases the risk of sudden death or incapacitation that the condition creates a danger to the safety and health of the driver, as well as to the public sharing the road. You are expected to assess the nature and severity of the medical condition and determine certification outcomes on a case-by-case basis and with knowledge of the demands of commercial driving. You should not certify the driver until the etiology is confirmed, and treatment has been shown to be adequate/effective, safe, and stable. As the medical examiner, your fundamental obligation during the medical assessment is to establish whether a driver has any disease or disorder that increases the risk for sudden death or incapacitation, thus endangering public safety. Additional questions should be asked, to supplement information requested on the form, to adequately assess medical fitness for duty of the driver. Advisory Criteria/Guidance Hernia the Medical Examination Report form physical examination section includes checking for hernia for both the abdomen and viscera body system and the genitourinary system. Decision Maximum certification - 2 years Recommend to certify if: As the medical examiner, you believe that the nature and severity of the medical condition of the driver does not endanger the safety and health of the driver and the public. Monitoring/Testing You may, on a case-by-case basis, obtain additional tests and/or consultation to adequately assess driver medical fitness for duty. Nephropathy Diabetic nephropathy accounts for a significant number of the new cases of end-stage renal disease. The first sign of nephropathy commonly is the development of persistent proteinuria. Whether nephropathy is a disqualifying factor should be determined on the basis of the degree of disease progression and the associated impact on driver ability to function. The prevalence of nephropathy is strongly related to the duration of diabetes mellitus. After 15 years of living with diabetes mellitus, the frequency of nephropathy is higher among individuals who use insulin than with individuals who do not use insulin. Waiting Period No recommended time frame You should not certify the driver until the etiology is confirmed, and treatment has been shown to be adequate/effective, safe, and stable. Has a treatment plan that manages the disease and does not interfere with safe driving. Recommend not to certify if: As the medical examiner, you believe that the nature and severity of the medical condition of the driver endangers the safety and health of the driver and the public. Monitoring/Testing Urinalysis - An abnormal urinalysis, including but not limited to proteinuria, may indicate some degree of renal dysfunction. When requesting additional evaluation from a specialist, the specialist must understand the role and function of a driver. Therefore, including copies of the Medical Examination Report form description of the driver role and the applicable medical standard(s) and guidelines with the request is helpful. You may require more frequent examinations, if indicated, to adequately monitor the progression of the condition. Proteinuria, hematuria, or glycosuria may be an indication for further testing to rule out any underlying medical problem. You should advise the driver of any abnormal findings and when indicated, encourage the driver to seek primary care provider evaluation, particularly if an abnormal urinalysis could indicate the presence of a medical condition that if left untreated could result in a serious illness that might affect driving. When an abnormal urinalysis is indicative of a medical condition that endangers the safety and health of the driver and the public, you should not certify the driver until the etiology is confirmed and treatment has been shown to be adequate/effective, safe, and stable.
The effect of garlic on saquinavir levels in the presence of ritonavir (as a pharmacokinetic enhancer) does not appear to have been studied. Effect of oral administration of crude aqueous extract of garlic on pharmacokinetic parameters of isoniazid and rifampicin in rabbits. Garlic + Paracetamol (Acetaminophen) Studies in healthy subjects found that garlic did not affect the pharmacokinetics of single-dose paracetamol to a clinically relevant extent. Clinical evidence A study in 16 healthy subjects found that the use of an aged garlic extract (approximately equivalent to 6 to 7 cloves of garlic daily) for 3 months had little effect on the metabolism of a single 1-g oral dose of paracetamol. The effect of diallyl sulfone 25 mg/kg was equivalent to that of the known antidote, acetylcysteine. Garlic kinetic effect on single-dose ritonavir was not clinically important, but this requires confirmation in a multiple-dose study. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Severe gastrointestinal toxicity with concomitant ingestion of ritonavir and garlic. He was also taking enalapril 20 mg, furosemide 40 mg and pravastatin 20 mg (dosage frequency not stated). This effect on platelet aggregation has, on at least two documented occasions, led to spontaneous bleeding in the absence of an anticoagulant. Importance and management Information about an adverse interaction between coumarin anticoagulants and garlic seems to be limited to these two reports, with warfarin and fluindione. Bearing in mind the wide-spread use of garlic and garlic products, the limited information from the review5 and the study with aged garlic extract,4 it seems most unlikely that garlic usually has any generally important interaction with anticoagulants. In addition, garlic may have some antiplatelet effects and, although there appear to be no clinical reports of an adverse interaction between garlic and antiplatelet drugs, it may be prudent to consider the potential for an increase in the severity of bleeding if garlic is given with anticoagulants. The impact of complementary and alternative medicine use on warfarin-related adverse outcomes. Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: a case report. Garlic + Rifampicin (Rifampin) the information regarding the use of garlic with rifampicin is based on experimental evidence only. Importance and management Evidence appears to be limited to this one study in animals. Nevertheless, what is known suggests that no changes in the dose of rifampicin are likely to be needed if it is also taken with garlic. G Garlic + Warfarin and related drugs An isolated report described increases in the anticoagulant effects of warfarin in two patients taking garlic supplements. Another report described a decrease in anticoagulant effects of fluindione in a patient taking garlic tablets. Ginger is a constituent of Trikatu, a medicine used in Ayurvedic medicine in a ratio of 1:1:1 with Piper nigrum and Piper longum, see pepper, page 313. Pharmacokinetics Detailed information on the pharmacokinetics of ginger in humans is scarce but what has been found, in animals, is that gingerol, a major constituent of ginger, is rapidly cleared from plasma and elimination by the liver is involved. Generally, ginger rhizomes contain volatile oils of which zingiberene and bisabolene are major components: zingerone, zingiberol, zingiberenol, curcumene, camphene and linalool are minor components. The rhizomes also contain gingerols and their derivatives, gingerdiols, gingerdiones and dihydrogingerdiones. Shogaols are formed from gingerols during drying, and together these make up the pungent principles of ginger. Ginger extracts have been standardised to contain a minimum of 15 mL/kg of essential oil with reference to the dried drug. Interactions overview There are isolated cases of ginger increasing the response to anticoagulant treatment with warfarin and related drugs, but a controlled study did not confirm an interaction. A small study showed antiplatelet effects for ginger that were synergistic with those of nifedipine, but any effect needs confirming. For the interactions of ginger as a constituent of Chinese herbal medicines, see under bupleurum, page 89. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. G Use and indications Ginger is thought to possess carminative, anti-emetic, antiinflammatory, antispasmodic and antiplatelet properties.
Understand the following functional categories of mutations: loss-of- function (inactivating) mutations, gain-of-function (activating) mutations, null mutations 6. Know linkage disequilibrium and describe how haplotype mapping aids identification of disease-causing genes c. Be able to describe chromosome abnormalities such as aneuploidy, small deletions, duplications, translocations, etc. Understand the importance of family studies to determine linkage phase of mutations detected in an individual with a genetic disease 2. Understand the concept of a dominant negative mutation and the mechanisms involved b. Be able to describe basic methodologies used to examine mechanisms of growth control at the cellular level, such as regulation of replication and apoptosis C. Know the principles of methods used for determining binding capacity and affinity of receptors b. Understand that liganded cell-surface receptors often aggregate, are internalized into endosomes, and then can be recycled to the cell surface. Know the principles involved and interpretation of results in radioreceptor assays 2. Know that phosphorylation of proteins by various classes kinases plays important functions in signal transduction b. Understand the roles of adenylate cyclase and phospholipase C in signal transduction. Understand that intracellular receptors in the steroid hormone receptor superfamily bind to hormone response elements in promoters and alter transcription of target genes f. Recognize the value of and techniques for measuring free and protein- bound concentrations of certain hormones. Understand that the lower and upper limit of diagnostic test range is defined by the 2nd and 98th percentiles, respectively, and thus that slightly abnormal measurements are unlikely to be clinically significant f. Understand the value of procedures such as extraction and chromatography to increase assay specificity g. Recognize the potential effect of heterophilic/anti-animal antibodies on immunoassays and know that antibody effects may differ depending on whether the immunoassay is competitive or immunometric 2. Know that radioimmunoassays are based on competitive inhibition of the binding of labeled hormone to antibody by unlabeled hormone contained in standards and unknown samples and the methods involved 2. Know that immunoradiometric assays involve two antibodies directed against the standard or unknown; the unlabeled antibody captures; and labeled antibody "signals" or quantitates the standard or unknown d. Know the basic steps involved in a high performance liquid chromatography/ tandem mass spectrometry assay of a steroid molecule E. Understand basic pharmacological parameters such as clearance, volume of distribution, half-life F. Understand why epidemiological association does not imply causality, and recognize the need for randomized controlled studies to confirm possible associations 12. Understand how the type of variable (eg, continuous, categorical, nominal) affects the choice of statistical test 2. Understand when to use and how to interpret tests comparing continuous variables between two groups (eg, t test, Mann Whitney U) c. Understand when to use and how to interpret regression analysis (eg, linear, logistic) b. Understand when to use and how to interpret survival analysis (eg, Kaplan Meier) 7. Recognize the importance of an independent "gold standard" in evaluating a diagnostic test b. Understand how disease prevalence affects the positive and negative predictive value of a test. Recognize and understand the strengths and limitations of a cohort study, case control study, and randomized controlled clinical trial b. Assess how the data source (eg, diaries, billing data, discharge diagnostic code) may affect study results 3. Understand factors that affect the rationale for screening for a condition or disease (eg, prevalence, test accuracy, risk benefit, disease burden, presence of a presymptomatic state) 7.
No apparent alterations in the risk of birth defects were found in C8 Health Studies (Darrow et al. A more in-depth discussion of the developmental toxicity of perfluoroalkyls in animals is included in Section 2. No relevant studies were located regarding interactions of perfluoroalkyl compounds with other chemicals in children or adults. No information was located regarding pediatric-specific methods for reducing peak absorption following exposure to perfluoroalkyl compounds, reducing body burden, or interfering with the mechanism of action for toxic effects. The available epidemiology data identify several potential targets of toxicity of perfluoroalkyls, and individuals with pre-existing conditions may be unusually susceptible. Thus, an increase in serum cholesterol may result in a greater health impact in individuals with high levels of cholesterol or with other existing cardiovascular risk factors. Similarly, increases in uric levels have been observed in individuals with higher perfluoroalkyl levels; increased uric acid may be associated with an increased risk of high blood pressure and individuals with hypertension may be at greater risk. The liver has been shown to be a sensitive target in a number of animal species and there is some indication that it is also a target in humans. Therefore, individuals with compromised liver function may represent a susceptible population. Likewise, individuals with a compromised immune system may have an increased risk of perfluoroalkyl-induced immunotoxicity. The preferred biomarkers of exposure are generally the substance itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. The National Report on Human Exposure to Environmental Chemicals provides an ongoing assessment of the exposure of a generalizable sample of the U. If available, biomonitoring data for perfluoroalkyls from this report are discussed in Section 5. They also may not be directly adverse, but can indicate potential health impairment. It can be an intrinsic genetic or other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the biologically effective dose, or a target tissue response. Although elevated serum levels are likely to be indicative of exposure to the parent compound, their presence in blood can also indicate exposure to other perfluoroalkyl compounds. Two studies have also evaluated the use of perfluoroalkyl levels in hair as a biomarker of exposure. The study did not evaluate the potential relationship between serum perfluoroalkyl levels and hair levels, which does not allow for an assessment of whether it is a viable biomarker of exposure. Particularly absent are studies examining toxicological and toxicokinetic interactions of a perfluoroalkyl compound with other perfluoroalkyl compounds. No additional information was located regarding interactions among chemicals of this class or between perfluoroalkyl compounds and other chemicals. Therefore, it is not unreasonable to speculate that interactions at the receptor level might occur; however, there are no experimental data to support or rule out this presumption. This information includes synonyms, chemical formulas and structures, and identification numbers. The perfluoroalkyls discussed in this profile exist as linear and branched isomers depending upon the method of production (see Chapter 5) and the reported values for the physical-chemical properties are typically reflective of the mixtures rather than a single specific isomer. Perfluoroalkyl compounds are very stable, owing to the strength of the carbon-fluorine bonds, the presence of the three electron pairs surrounding each fluorine atom, and the shielding of the carbon atoms by the fluorine atoms (3M 1999; Kissa 2001; Schultz et al. Perfluoroalkyl carboxylates and sulfonates consist of a perfluorocarbon tail that is both hydrophobic and oleophobic and a charged end that is hydrophilic (3M 1999; de Vos et al. This combination of hydrophobic and oleophobic characteristics makes these substances very useful as surfactants. The ability of these substances to repel oil, fat, and water has resulted in their use in surface protectants (Kissa 2001). Their ability to reduce the surface tension of aqueous systems to <20 mN/m has resulted in their use as wetting agents (Kissa 2001). Neutral or uncharged perfluoroalkyls or very long chain constituents are expected to form separate layers when mixed with hydrocarbons and water.