"Buy cheap telmisartan 80 mg on-line, arrhythmia interpretation".
G. Uruk, MD
Clinical Director, Des Moines University College of Osteopathic Medicine
Lesions rarely may cause problems because of size and obstruction, but the biggest problems are cosmetic and psychosocial. Testing methods for epidemiologic surveillance differ from those used in clinical applications. Epidemiologic methods require type-specific assays with high analytic sensitivity. Use in clinical settings requires either that laboratories rely on assays that have been validated by regulatory agencies for specific clinical indications, or that laboratories undertake validation of the clinical performance of the assay. Moreover, in combination with increased expression of other host factors such as Ki67, overexpression of p16 correlates well with transforming viral infections. Clinical applications now rely on highly standardized assays that streamline sample handling and rely on some form of amplification for testing. In addition, a low-cost version has been designed to be performed in low-resource settings requiring minimal equipment and training and operating as a rapid assay (43). Source: Reprinted with permission from Magnus von Knebel Doeberitz, Department of Applied Tumor Biology, Institute of Pathology, University of Heidelberg, Heidelberg, Germany. Source: Reprinted with permission from the American Journal of Surgical Pathology. Appropriate handling for swabs includes transporting dry or in a viral transport medium, whereas scrapes and biopsy should be collected in viral transport medium. Analytics Following specimen collection, extraction or release of nucleic acid from samples must occur. Inclusion of positive and negative cell line controls can be useful in monitoring results. Processing water blanks through all steps of the assay are particularly crucial for monitoring false-positive results that may occur through crosssample contamination. Clinically approved assays include guidelines for monitoring and reporting assays. Well verified, in-house, laboratory-developed assays can also be used in routine diagnostics. Where an approved commercial assay is available, it is recommended by accrediting authorities that the latter should be utilized. Some of the advantages of doing so are that commercial assays usually include quality controlled reagents, as well as appropriate controls. Principles of these various molecular assays are described under the following headings (51): Laboratory quality assurance Good microbiological practices should be followed, including appropriate positive and negative controls. Preanalytics Suitable specimens and appropriate handling of clinical samples are essential in obtaining an accurate result. It is important to utilize appropriate tests whether it be for clinical applications or epidemiological purposes. Clinical tests require careful standardization of sample collection, processing, and testing, with correlation to clinical (not analytic) endpoints. Ongoing research is investigating the usefulness of certain progression markers to improve specificity for clinically important outcomes. Human papillomavirus biology and cervical neoplasia: implications for diagnostic criteria and testing. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Phylogenetic classification of human papillomaviruses: correlation with clinical manifestations. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets.
If barriers are breached (almost inevitably), astronauts must be equipped with the knowledge and resources to institute specific emergency response plans on their own. This means that interchange between the martian environment and the terran-like environments that the astronauts bring with them would be an inevitability. Second, there is the possibility for dust with unknown toxicity to be inhaled or ingested by the crew, with similarly unknown potential for acute and chronic effects on their health status. Third, the dust is everywhere and, similarly to Apollo [3] will represent a challenge for habitation exploration systems and act as a carrier (in and out) of microbial life, both Terran and Extraterrestrial (should there be any). Planetary Protection as an Issue for Human Exploration: the international consensus goals for planetary protection in the Outer Space Treaty (to which all spacefaring nations are signatories) are expressed as: "The conduct of scientific investigations of possible extraterrestrial life forms, precursors, and remnants must not be jeopardized. In addition, the Earth must be protected from the potential hazard posed by extraterrestrial matter carried by a spacecraft returning from another planet or other extraterrestrial sources" [4]. While detailed approaches, implementations and requirements to achieve this are mature for robotic missions [5], only high-level guidelines are available for how planetary protection might be implemented on crewed missions. More information is needed before the following dust-related factors can be addressed in a planetary protection risk assessment, and adequate mitigations be identified and deployed. This finding holds true even on the undersides of spacecraft, but under protective layers of dust, spores could reamin almost indefinitely, waiting for conditions to change that would allow them to replicate. This could be due to dust deposition onto spacecraft hardware, or onto the martain surface where contaminant organisms had been released from a spacecraft hardware element or crewmember. Dust to Breathe and Ingest: On the issue of astronaut health, it has to be considered what the impact of a ~500 day exposure to martian material would be. Based on the properties of dust we have seen displayed during robotic explorations [7] and the chemistry of the dust, some ingestion of perchlorates by astronauts would seem to be an extremely likely scenario. Goiter, caused by iodine depletion, is the most well-known ailment on Earth that might result from exposure to a perchlorate-rich environement like Mars. As well as swelling in the thyroid, it can also be responsible for fatigue, weight gain and depression in sufferers, with severe cases resulting in serious mental health problems, brain damage and death. As well as perchlorate, chemical constituents such as hexavalent chromium (or any other toxic trace contaminant) that may be present in the martian environment could cause illness that may be indistinguishable based on present information from effects of exposure to a martian organism. Dust as an Environmental Constant: For the Apollo crews dust was a significant contributor to equipment failures, even after stays of only a couple of days. A preliminary lab study by Mancinelli [8] indicated that microorganisms would be shielded by lofted dust. However, sufficient protection may only occur on relatively large lofted grains (~3-45 m), rather than on the smaller grains (<~3 m) that comprise large dust storms on Mars. Larger grains are not typically transported far from their source regions, so that locally-lifted particles may be the most important contribution to forward contamination. However, quantitation of these parameters (size and bioload of contaminated dusts) is needed before quantitative assessment of planetary protection risk can be made. First, there is the threat of dust contaminated by terrestrial organisms being released and dispersed in the martian environment. Next is the threat that astronauts, affected by the Mars environment, might be unable to ascertain whether their ailment is simply due to exposure to chemical irritants or due to exposure to an environmentally-encountered martian organism. First, a significantly enhanced knowledge of the martian environment and its effects, particularly the dust environment, its chemistry and habitability is required. Second, a more complete understanding of the release, processes and fate affecting terrestrial biota introduced into the martian dust environment is required. Third, the performance requirements for the systems and operations of the mission architecture is needed to appropriately control the ingress and egress threat of martian and terrestrial material, respectively. Utilizing the extensive experience gained from the Stardust and Genesis missions, these samples would then be returned to Earth. This mission would collect a suite of samples that is distinct from that currently being planned for other sample return missions that are under consideration. As highlighted in the most recent National Academies Planetary Science Decadal Survey [1], the next major step in Mars exploration is returning samples to terrestrial laboratories for analysis, where the variety and precision of measurements far exceed practical in situ or remote sensing capability. Sample return missions enable the analytic capability required to achieve highpriority science as defined by the science community as well as to address concerns specific to future human exploration. Surface sample return missions to Mars are necessarily faced with significant challenges of entry, descent, landing, surface operations, followed by launch and orbit rendezvous, and planetary protection, which result in high mission risk as well as cost.
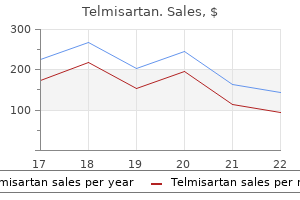
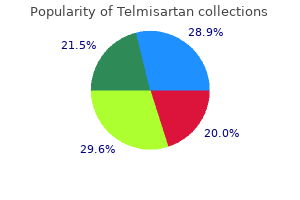
Provide clinical history, age of patient, relevant vaccination history, and specimen collection date. Whenever possible, submit both acute and convalescent sera from patients for whom virus isolation tests are being requested. Continued Next Page> Specimen Rejection Criteria: Availability: Results and Interpretation: Additional Information: Guide to Public Health Laboratory Services December 2020 edition v2. Mehsen Joseph Public Health Laboratory Purpose of Test: Method: Interfering Substances: Testing Site: Comment: Virus isolation to determine probable cause of infection and aid in the diagnosis of viral disease or to further characterization for epidemiological purposes. Please indicate suspected infecting agent as well as additional information such as chief symptoms, clinical test results, epidemiology data, immunizations, etc. For example, isolation of a virus from the brain in encephalitis or from the spinal fluid in aseptic meningitis provides direct evidence of an etiological association. Occasionally a virus other than the one ordered is detected since any reaction in the host system is investigated. Transport Conditions: Store and ship at room temperature, ship as quickly as possible. Continued Next Page> Page 117 of 138 Guide to Public Health Laboratory Services December 2020 edition v2. Specimens must be packaged in a triple packaging system to ensure that under normal conditions of transport they cannot break, be punctured or leak their contents (Refer to pages 9 & 10 for triple packing guidance). Grossly hemolyzed specimens, unlabeled specimen, leaking container, duplicate specimen type. Specimen Volume (Optimum): Specimen Volume (Minimum): Collect: Request Form: Packaging and Shipping*: Transport Conditions: Specimen Rejection Criteria: Availability: Results and Interpretation: Additional Information: Purpose of Test: Method: Interfering Substances: Store refrigerated and ship on cold packs in a cooler. A positive IgG antibody and a negative IgM antibody are consistent with infection in the distant past and are not consistent with acute infection. Specimen Volume (Optimum): Specimen Volume (Minimum): Collect: Request Form: Packaging and Shipping*: Transport Conditions: Specimen Rejection Criteria: Availability: Results and Interpretation: Store refrigerated and ship on cold packs in a cooler. Continued Next Page> Page 120 of 138 Guide to Public Health Laboratory Services December 2020 edition v2. Sputum may be examined but this is not advised because of contamination by normal throat flora. Aspirate of involved tissue (bubonic) or biopsied specimen: Liver, spleen, bone marrow, lung. Syringe and needle of aspirated sample should be capped, secured by tape, and sent to the Laboratory. Turnaround Time [from specimen receipt in the Laboratory]: Specimen Required: Specimen Identification: Specimen Volume (Optimum): Specimen Volume (Minimum): Guide to Public Health Laboratory Services December 2020 edition v2. Transport Conditions: Specimen Rejection Criteria: Availability: Results and Interpretation: Additional Information: Purpose of Test: Method: Interfering Substances: Testing Site: Comment: Respiratory/sputum: Transport at room temperature. Tissue aspirate/biopsy specimen: Transport the sample at room temperature for immediate processing. Unlabeled or improperly labeled specimen Non-sterile or leaking container Inappropriate specimen transport conditions Illegible, or no submitter information on the request form Mismatched form and specimen Broken specimen/sample container the wrong specimen for test request Inappropriate outfit for requested test Illegible or no patient information on the specimen Expired transport media 24 hours/day, 7 days/week Yersinia pestis isolated/detected Yersinia pestis not found Call 410-925-3121 before sending to the Laboratory. If indicated, please submit another serum specimen collected greater than 14 days after onset of illness for further testing. Other Flavivirus Positive: Specimen tested presumptively positive for IgM antibody to another flavivirus. There still may be low levels of Zika IgM antibody present and follow up testing is required; the possibility of co-infections must also be considered. Virus specific IgM antibodies can be detectable equal to or greater than four days after onset of illness. It has been reported that IgM antibodies typically persist for approximately 2-12 weeks.
In the closing days of the program, researchers initiated the first experiments in metabolic engineering as a means of increasing oil production. These early experiments did not, however, demonstrate increased oil production in the cells. Algae Production Systems Demonstration of Open Pond Systems for Mass Production of Microalgae. Over the course of the program, efforts were made to establish the feasibility of large-scale algae production in open ponds. Based on results from six years of tests run in parallel in California and Hawaii, 1,000 m2 pond systems were built and tested in Roswell, New Mexico. The Roswell test site successfully completed a full year of operation with reasonable control of the algal species grown. Single day productivities reported over the course of one year were as high as 50 grams of algae per square meter per day, a long-term target for the program. Attempts to achieve consistently high productivities were hampered by low temperature conditions encountered at the site. The desert conditions of New Mexico provided ample sunlight, but temperatures regularly reached low levels (especially at night). If such locations are to be used in the future, some form of temperature control with enclosure of the ponds may well be required. An important lesson from the outdoor testing of algae production systems is the inability to maintain laboratory organisms in the field. Algal species that looked very promising when tested in the laboratory were not robust under conditions encountered in the field. In fact, the best approach for successful cultivation of a consistent species of algae was to allow a contaminant native to the area to take over the ponds. A major conclusion from these analyses is that there is little prospect for any alternatives to the open pond designs, given the low cost requirements associated with fuel production. These analyses point to the need for highly productive organisms capable of near-theoretical levels of conversion of sunlight to biomass. Even with aggressive assumptions about biological productivity, we project costs for biodiesel which are two times higher than current petroleum diesel fuel costs. Such resource assessments require a combined evaluation of appropriate climate, land and resource availability. Algal biodiesel could easily supply several "quads" of biodiesel-substantially more than existing oilseed crops could provide. In order to provide a consistent context and framework for understanding this detail, we have attempted to outline the major activities of the program as they unfolded over the course of the past two decades. The timeline on the following page shows the major activities broken down into two main categories-laboratory studies and outdoor testing/systems analysis. For the sake of clarity and brevity, many of the research projects and findings from the program are not presented here. Instead, only those findings that form a thread throughout the work are highlighted. Biochemical and physiological studies of lipid production Molecular biology and genetic engineering studies 13 A Look Back at the Aquatic Species Program-Program Summary There is a logic to the sequence of these activities. Researchers first identified a need to collect and identify algae that met minimal requirements for this technology. Once a substantial amount of information was available on the types of oil-producing algae and their capabilities, the program began to switch its emphasis to understanding the biochemistry and physiology of oil production in algae. A natural next step was to use this information to identify approaches to genetically manipulate the metabolism of algae to enhance oil production. Algae collection efforts initially focused on shallow, inland saline habitats, particularly in western Colorado, New Mexico and Utah.