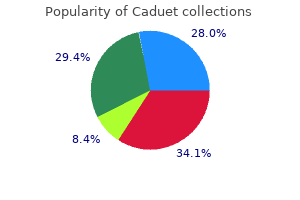
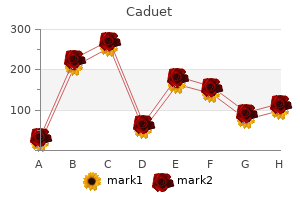
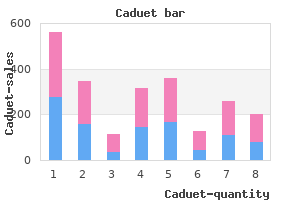
"Discount caduet 5mg visa, cholesterol levels blood pressure".
A. Rune, M.A., M.D., M.P.H.
Clinical Director, Stony Brook University School of Medicine
All infants show a slight decline of serum calcium levels after birth, reaching a trough level at 24 to 48 hours, the point at which hypocalcemia usually occurs. Total serum calcium levels of less than 7 mg/dL and ionized calcium levels of less than 3 to 3. The etiology of hypocalcemia varies with the time of onset and the associated illnesses of the child. Early neonatal hypocalcemia occurs in the first 3 days of life and is often asymptomatic. Transient hypoparathyroidism and a reduced parathyroid response to the usual postnatal decline of serum calcium levels may be responsible for hypocalcemia in premature infants and infants of diabetic mothers. Congenital absences of the parathyroid gland with DiGeorge syndrome is a cause of hypocalcemia. Treatment with calcium alone does not relieve symptoms or increase serum calcium levels until hypomagnesemia is also treated. Sodium bicarbonate therapy, phosphate release from cell necrosis, transient hypoparathyroidism, and hypercalcitoninemia may be responsible for early neonatal hypocalcemia associated with asphyxia. Early-onset hypocalcemia associated with asphyxia often occurs with seizures as a result of hypoxic-ischemic encephalopathy Late neonatal hypocalcemia, or neonatal tetany, often is the result of ingestion of high phosphateontaining milk or the inability to excrete the usual phosphorus in commercial infant formula. Hyperphosphatemia (>8 mg/dL) usually occurs in infants with hypocalcemia after the first week of life. Vitamin D deficiency states and malabsorption also have been associated with late-onset hypocalcemia. The clinical manifestations of hypocalcemia and hypomagnesemia include apnea, muscle twitching, seizures, laryngospasm, Chvostek sign (facial muscle spasm when the side of the face over the seventh nerve is tapped), and Trousseau sign (carpopedal spasm induced by partial inflation of a blood pressure cuff). Early asymptomatic hypocalcemia of preterm infants and infants of diabetic mothers often resolves spontaneously. Symptomatic hypocalcemia should be treated with 2 to 4 mL/kg of 10% calcium gluconate given intravenously and slowly over 10 to 15 minutes, followed by a continuous infusion of 75 mg/kg/24 hr of elemental calcium. Neonatal Drug Addiction and Withdrawal Infants may become passively and physiologically addicted to medications or to drugs of abuse (heroin, methadone, barbiturates, tranquilizers, amphetamines) taken chronically by the mother during pregnancy; these infants subsequently may have signs and symptoms of drug withdrawal. Opiates Neonatal withdrawal signs and symptoms usually begin at 1 to 5 days of life with maternal heroin use and at 1 to 4 weeks with maternal methadone addiction. Clinical manifestations of withdrawal include sneezing, yawning, ravenous appetite, emesis, diarrhea, fever, diaphoresis, tachypnea, high-pitched cry, tremors, jitteriness, poor sleep, poor feeding, and seizures. When hyperactivity is constant, and irritability interferes with sleeping and feeding, or when diarrhea or seizures are present, pharmacologic treatment is indicated. The other symptoms may be managed with replacement doses of a narcotic (usually tincture of opium) to calm the infant; weaning from narcotics may be prolonged over 1 to 2 months. Cocaine Cocaine use during pregnancy is associated with preterm labor, abruptio placentae, neonatal irritability, and decreased attentiveness. Autoantibody-mediated diseases can have direct consequences on the fetus and neonate because the antibodies are usually of the IgG type and can cross the placenta to the fetal circulation. Antiphospholipid antibodies are found in 2% to 5% of the general healthy population, but they also may be associated with systemic lupus erythematosus and other rheumatic diseases. Obstetric complications arise from the prothrombotic effects of the antiphospholipid antibodies on placental function. Vasculopathy, infarction, and thrombosis have been identified in mothers with antiphospholipid syndrome. Antiphospholipid syndrome can include fetal growth impairment, placental insufficiency, maternal preeclampsia, and premature birth. For infants who have evidence of hemorrhage, single-donor irradiated platelets may be administered to control the bleeding. Plateletassociated IgG antibodies can cross the placenta and cause thrombocytopenia in the fetus and newborn. The severely thrombocytopenic fetus is at increased risk for intracranial hemorrhage. The most serious complication is damage to the cardiac conducting system, which results in congenital heart block. Neonatal lupus may occur and is characterized by skin lesions (sharply demarcated erythematous plaques or central atrophic macules with peripheral scaling with predilection for the eyes, face, and scalp), thrombocytopenia, autoimmune hemolysis, and hepatic involvement. The prevalence of clinical hyperthyroidism in pregnancy has been reported to be about 0.
Syndromes
- Percutaneous transhepatic cholangiogram (PTCA)
- The time it was swallowed
- A fertilized egg or embryo does not survive once it sticks to the lining of the womb (uterus)
- Fainting or feeling light-headed
- Neurological examination
- Do NOT push or pick debris from a wound. Seek medical attention.
- Drug-induced lupus erythematosus
- Any condition that blocks the flow of cerebrospinal fluid (CSF)
With the magnetic stimulator, supramaximal root stimulation can usually not be achieved. For lower limb studies, magnetic stimulation of the lumbosacral roots is not always satisfactorily possible, necessitating high-voltage electrical stimulation (see. For the mere purpose of estimating a peripheral conduction time, supramaximal stimulation of the motor roots is not needed. The actual site of intracranial facial nerve stimulation can be localized to the inner part of the facial petrosal canal,16 hence the term canalicular stimulation. For this canalicular stimulation, the optimal coil position and orientation is distinct from that of cortical facial area stimulation: the stimulating coil is placed ipsilateral to the target nerve, preferably posterior to the ear with the inducing current in the relevant coil segment flowing anterolaterally. However, the central motor conduction time assessed in such a way still comprises a short peripheral segment of the facial nerve, the portion between exit from brain stem and entrance into the petrosal (fallopian) canal. Although the exact stimulator and coil type is not so critical, it is important to use the same method of assessing peripheral conduction time. Magnetic and high-voltage electrical root stimulation provide similar latencies at cervical level as long as not very high electrical currents are used in an attempt to stimulate supramaximally. Supramaximal stimulation is however essential when amplitude measurements are used. At the lumbosacral level, magnetic stimulation often does not suffice and an electrical high-voltage device is preferred. Normal values of the total corticomuscular latency are preferably related to the arm length (upper limbs) or height (lower limbs). In practice, the stimulus intensity is increased at 5% steps until reaching a level that induces approximately 100-V responses in about 50% of 10 consecutive trials. The relatively high corticomotor threshold of lower limb muscles in elderly subjects must be taken into account, necessitating maximum stimulator output and thorough search for the optimal coil placement on the scalp before an absent response can be taken as abnormal. In case of conduction failure of these large fibers, transmission may occur by small, slowly conducting fibers or by some alternative, oligosynaptic pathway, such as the corticorubrospinal tracts. This mechanism may account for the great delays sometimes encountered in axonal and demyelinating (conduction block) disorders. The rapidly conducting corticospinal neurons use high frequency activity (about 600 to 800 Hz) to excite bulbar and spinal neurons, and they are known to send short descending volleys in response to a transcranial cortical stimulus. The spinal motoneurons, particularly the larger ones, require much excitatory input to reach firing threshold. Drop-out of corticospinal fibers reduces the spatial summation of excitatory input on spinal motoneurons, necessitating more temporal summation to get the spinal motoneurons to discharge. It is likely that fewer functioning corticospinal neurons take longer to get the spinal motoneurons to fire (if at all) in response to the impinging excitatory burst. A similar delay can theoretically also result from pathological hypoexcitability of spinal motoneurons. Whether this mechanism comes into play very frequently (which may be the case) and whether it also operates Central Motor Conduction and Its Clinical Application 91 when using the F-wave technique to assess peripheral conduction is unknown. In a much dispersed biphasic response, cancellation phenomena of the negative and positive deflections further abate the amplitude. Because pure slowing of conduction due to demyelination increases temporal dispersion, further attenuation of amplitude may result without drop-out of active fibers. Demyelinating lesions can cause conduction block, which also results in drop-out of active fibers. However, demyelinating diseases probably always comprise a certain degree of axonal loss as well. Using the triple-stimulation technique, it was also shown that, irrespective of the precise mechanism. Central Motor Conduction and Its Clinical Application 93 related to functional motor disability, which has been amply confirmed since. Depending on the precise method used, subclinical involvement was detected in 4% to 13.
This principle is intuitively obvious, but it underlies the necessity for preoperative discussions between the surgeon and neurophysiologist, particularly when the patient has preexisting pathology or the operative procedure is not one for which monitoring has previously been requested. It is particularly important to assess transmission at or across the likely site of intraoperative dysfunction. The identification of abnormal function should occur with sufficient time for the surgeon to do something about the dysfunction. The ideal technique would provide ``real-timefeedback,' but this situation cannot apply when the stimulus repetition rate is low, averaging is required to define the evoked volley, and the putative abnormality must be reproduced to show that it is not due to noise or chance variation in waveforms. In practice, however, it often takes less time to record duplicate averages than to convince the surgeon that something is amiss and that surgery needs to be interrupted, reversed, or even aborted. Intraoperative Monitoring of Corticospinal Function 3 69 g Stimulation Site of Stimulation in the Central Nervous System Although this chapter focuses on transcranial stimulation, there are circumstances when stimulation at other sites may be valuable, such as when the operation is on the low spinal cord, cauda equina, or nerve roots. With spinal stimulation, many spinal tracts, both descending and ascending, may be activated, and there is evidence that the optimal stimulus parameters for pulse trains are not the same as with transcranial stimulation. For example, the 2-ms interval has been recommended by advocates of spinal stimulation, but longer intervals appear to be preferable for transcranial stimulation. Although the cathode is posterior to the cord, collision studies have demonstrated that the corticospinal pathways are activated by spinal cord stimulation at the threshold for the evoked spinal cord volley. The major use of the evoked spinal cord volley is that it may allow a monitoring service to be offered when preexisting disease renders conventional monitoring techniques of little use. Similarly, the corticospinal system can be accessed at the decussation of the pyramids (where there is a bend in the axons) by transpalatal or transmastoid stimulation. Collision studies have demonstrated that stimuli delivered at this site access corticospinal axons and may do so almost exclusively. However, this benefit is neutralized by the changes that occur in the response to magnetic stimulation when patients are anesthetized. Both forms of stimulation ultimately produce a current flow that depolarizes axons; they differ merely in the method of delivering that current flux to neural structures. The term magneto-electrical has been used instead of magnetic merely to reflect this fact. The stimulating coil intrudes more into the anesthetic field, and the activities of the anesthetist may displace it. With operations on the brain and brainstem, the coil intrudes more into the operative field. The coil may heat up with repetitive use, and excessive heating may cause the machine to be disabled temporarily, interrupting monitoring, something that would be undesirable during hazardous surgery. These problems constituted important limitations on the use of older stimulators, but both have been addressed with current-generation stimulator design. However, when recording corticospinal volleys using epidural electrodes, the magnetic volley will have greater variability than the electrical volley. However, the only real limitation of transcranial electrical stimulation is pain experienced by conscious patients. However, with the stimulus intensities used for monitoring, this is more likely to have affected the ability to stimulate corticocortical axons, activity of which is responsible for I waves. Access to corticospinal axons was probably little affected by the location of the cathode, and it is likely that the D wave involved volleys from both motor cortices. However, we have the impression that this has resulted in a greater stimulus artifact in the epidural recordings. Stimulating Electrodes Site of Stimulating Electrodes With transcranial electrical stimulation, the threshold for a corticospinal volley is lower for anodal stimulation than cathodal, much as has been shown to be the case with direct stimulation of the motor cortex in animals. However, it is uncertain whether it explains the greater efficacy of anodal stimulation when the pyramidal cell is orientated horizontally relative to the scalp, as would be the case with lower limb areas of motor cortex. In the initial studies from this Unit, the anode was sited at the vertex and the cathode some 7 cm laterally, approximately over the hand area of motor cortex. The data in Figure 25 were recorded with this montage, which produces larger volleys for less stimulus artifact than a cathode at a more anterior site. Theoretically, the needle electrodes could result in a more focused current density in the scalp: in practice, there have been no untoward side effects with their use. Single Stimuli orTrains of Stimuli When recording epidural volleys, single stimuli are appropriate, the recommended repetition rate being approximately once every 3 seconds.
The cases which are of clinical importance are those complicating childbirth and abortion. The condition causes symptoms at the beginning of the second week when the patient complains of pain in the hypogastrium and back. The inflammation of the pelvic cellular tissue leads to the development of a large indurated swelling in the pelvis. In the early stages, the uterus is pushed to the opposite side and the indurated swelling of the parametrium extends from the uterus to the lateral wall of the pelvis, and fixes the uterus in the pelvis. It is impossible to separate the uterus from the swelling, because the parametrium extends to the wall of the uterus. On rare occasions, the effusion may point in the perinephric region, in the ischiorectal fossa and even in the buttock, having tracked through the greater sciatic foramen. Suppuration in parametric effusion is uncommon, and even if the effusion points and has to be incised, it is rare for frank pus to be evacuated. As the effusion is extraperitoneal, symptoms of peritoneal irritation are absent, but rectal symptoms may arise as the result of inflammation involving the rectum. Most parametric effusions subside under conservative antimicrobial treatment, but they are followed by scarring of the parametrium and this causes chronic pelvic pain. The scarred tissue draws the uterus over to the Tumours of the Fallopian Tubes Neoplasms of the fallopian tubes are extremely rare and often malignant. Affections of the Broad Ligament and Parametrium Haematoma Haematoma of the broad ligament and parametrium may result from ectopic gestation which ruptures extraperitoneally Figure 31. Chapter 31 Disorders of the Broad Ligament, Fallopian Tubes and Parametrium affected side and the thick scar tissue is readily palpated on bimanual examination. This clinical syndrome is especially common if the responsible organism is the anaerobic Streptococcus. Almost all parametritic effusions lie lateral to the uterus and vagina, where the parametrium is most plentiful. However, on rare occasions, an anteroposterior parametritis develops situated between the cervix and the rectal wall posteriorly, and the bladder and urethra anteriorly. The treatment of parametritis consists of bed rest, local heat and a full course of the appropriate antibiotic-similar to that described in the treatment of acute salpingo-oophoritis. Retroperitoneal Tumours Retroperitoneal tumours are included here because they are often mistaken for an ovarian tumour or a broad ligament tumour, and their exact nature is revealed only at laparotomy. These tumours are classified as: n n n n Congenital: Ectopic pelvic kidney should be suspected when a fixed pelvic mass is associated with the absence or malformation of the genital tract. Tumours of neurogenic origin, neurofibromas and tumours arising from the spinal meninges. Tumours of the Broad Ligament and Parametrium Myoma the most common tumour is a myoma. It may be primary, when it arises from the uterosacral or round ligament, and tissues in the broad ligament, or secondary, when it arises low in the lateral wall of the uterus or the cervix but grows laterally between the two layers of the broad ligament. In the latter, the myoma retains its attachment to the uterus, and the uterine vessels as well as the ureter lie lateral to the tumour. In case of a primary myoma, the uterine vessel is medial to the tumour, but the ureter may lie anywhere in relation to it though usually it is beneath the tumour. Primary myoma is also known as true broad ligament myoma and secondary myoma as false broad ligament tumour. When faced with a retroperitoneal tumour, the most thorough pre-operative investigations, viz. Two dangers are encountered during removal of the retroperitoneal tumour: n n the ureter may be close to the tumour and be cut or ligated unless it is identified at the start of the surgery. Large vessels of the hypogastric system may obtrude into the operative fields and these must be secured.