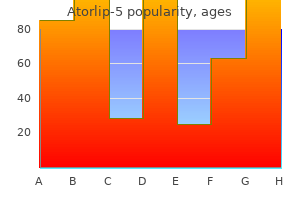
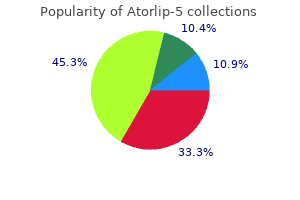
"Order atorlip-5, cholesterol levels garlic".
G. Berek, M.A., Ph.D.
Co-Director, Medical University of South Carolina College of Medicine
They do not act on cholinoceptors or adrenoceptors but have powerful pharmacologic effects on smooth muscle and other tissues. They are included in this chapter because of their effects on serotonin receptors and on smooth muscle. Excess production of histamine in the body (eg, in systemic mastocytosis) can be detected by measurement of its major metabolite, imidazole acetic acid, in the urine. Because it is released from mast cells in response to IgE-mediated (immediate) allergic reactions, this autacoid plays a pathophysiologic role in seasonal rhinitis (hay fever), urticaria, and angioneurotic edema. The triple response, a classic demonstration of histamine effect, is mediated mainly by H1 and H2 receptors. This response involves a small red spot at the center of an intradermal injection of histamine surrounded by an edematous wheal, which is surrounded by a red flare. H1 receptor-This Gq-coupled receptor is important in smooth muscle effects, especially those caused by IgE-mediated responses. Typical responses include pain and itching in the skin, bronchoconstriction, and vasodilation, the latter caused by histamine-evoked release of nitric oxide. H2 receptor-This Gs-coupled receptor mediates gastric acid secretion by parietal cells in the stomach. A third action is to reduce histamine release from mast cells-a negative feedback effect. In the periphery, it appears to be a presynaptic heteroreceptor with modulatory effects on the release of other transmitters (see Chapter 6). H4 receptor-The H4 receptor is located on leukocytes (especially eosinophils) and mast cells and is involved in chemotactic responses by these cells. Clinical Use Histamine has no therapeutic applications, but drugs that block its effects at H1 and at H2 receptors are very important in clinical medicine. Mechanism and Effects H1 blockers are competitive pharmacologic antagonists or inverse agonists at the H1 receptor; these drugs have no effect on histamine release from storage sites. Because their structure closely resembles that of muscarinic blockers and -adrenoceptor blockers, many of the first-generation agents are potent pharmacologic antagonists at these autonomic receptors. As noted, most older first-generation agents are sedating, and some-not all-first-generation agents have anti-motion sickness effects. Clinical Use H1 blockers have major applications in allergies of the immediate type (ie, those caused by antigens acting on IgE antibody-sensitized mast cells). Diphenhydramine, dimenhydrinate, cyclizine, meclizine, and promethazine are used as anti-motion sickness drugs. Doxylamine, in combination with pyridoxine, is promoted for the prevention of morning sickness in pregnancy. Toxicity and Interactions Sedation is common, especially with diphenhydramine and promethazine and these drugs should not be consumed before operating machinery. Antimuscarinic effects such as dry mouth and blurred vision occur with some firstgeneration drugs in some patients. Alpha-adrenoceptor blockade, which is significant with phenothiazine derivatives such as promethazine, may cause orthostatic hypotension. Interactions occur between older antihistamines and other drugs with sedative effects (eg, benzodiazepines and alcohol). Drugs that inhibit hepatic metabolism may result in dangerously high levels of certain antihistaminic drugs that are taken concurrently. Classification and Prototypes A wide variety of antihistaminic H1 blockers are available from several different chemical families. The older members of the first-generation agents, typified by diphenhydramine, are highly sedating agents with significant autonomic receptor-blocking effects. A newer subgroup of first-generation agents is less sedating and has much less autonomic effect.
Most of the free drug in the gut, including that released by deconjugation of glucuronides by enteric bacteria is reabsorbed (enterohepatic cycling) and ultimate excretion occurs in urine. Drugs that attain high concentrations in bile are erythromycin, ampicillin, rifampin, tetracycline, oral contraceptives, vecuronium, phenolphthalein. Exhaled air Gases and volatile liquids (general anaesthetics, alcohol) are eliminated by lungs, irrespective of their lipid solubility. Lungs also serve to trap and extrude any particulate matter that enters circulation. Most of the saliva along with the drug in it, is swallowed and meets the same fate as orally taken drug. Milk the excretion of drug in milk is not important for the mother, but the suckling infant inadvertently receives the drug. The released D is reabsorbed from the gut to again reach the liver through portal circulation and complete the enterohepatic cycle. Nevertheless, it is advisable to administer any drug to a lactating woman only when essential. Drugs that are safe, as well as those contraindicated during breast feeding or need special caution are given in Appendix-4 at the end of the book. The amount of drug or its metabolites ultimately present in urine is the sum total of glomerular filtration, tubular reabsorption and tubular secretion. Tubular reabsorption this occurs by passive diffusion and depends on lipid solubility and ionization of the drug at the existing urinary pH. Lipid-soluble drugs filtered at the glomerulus back diffuse in the tubules because 99% of glomerular filtrate is reabsorbed, but nonlipid-soluble and highly ionized drugs are unable to do so. This principle is utilized for facilitating elimination of the drug in poisoning. Though elimination of weak bases (morphine, amphetamine) can be enhanced by acidifying urine, this is not practiced clinically, because acidosis can induce rhabdomyolysis, cardiotoxicity and actually worsen outcome. The effect of changes in urinary pH on drug excretion is greatest for those having pKa values between 5 to 8, because only in their case pH dependent passive reabsorption is significant. Active transport of the drug across tubules reduces concentration of its free form in the tubular vessels and promotes dissociation of protein bound drug, which then becomes available for secretion. Thus, protein binding, which is a hinderance for glomerular filtration of the drug, is not so (may even be facilitatory) to excretion by tubular secretion. However, for drugs and their metabolites (exogenous substances) secretion into the tubular lumen predominates, whereas an endogenous substrate like uric acid is predominantly reabsorbed. This is because penicillin is primarily secreted while uric acid is primarily reabsorbed. Renal function again progressively declines after the age of 50 years; renal clearance of most drugs is substantially lower in the elderly (>75 yr). A fraction of the drug molecules present in plasma are removed on each passage through the organs of elimination. Most drugs infact have multicompartment distribution and multiexponential decay of plasma concentration-time plot. Half-lives calculated from the terminal slopes (when plasma concentrations are very low) are exceptionally long, probably due to release of the drug from slow equilibrating tissues, enterohepatic circulation, etc. This applies to majority of drugs which do not saturate the elimination processes (transporters, enzymes, blood flow, etc. However, if the dose is high enough, elimination pathways of all drugs will get saturated. As a result plasma concentration increases disproportionately with increase in dose.
The available preparations may be roughly graded as: Potent Beclomethasone dipropionate Betamethasone benzoate Betamethasone valerate Halcinonide Clobetasol propionate Dexamethasone sod. Areas of high penetration easily develop adverse effects-potent preparations should be avoided. Lotions and creams (to some extent) are better for exudative lesions-they allow evaporation, have a cooling, drying and antipruritic effect. Very potent preparations should be restricted to severe inflammatory conditions, unresponsive eczema, psoriasis, etc. Local adverse effects of topical steroids Thinning of epidermis Dermal changes-atrophy Telangiectasia, Striae Easy bruising Hypopigmentation Delayed wound healing Fungal and bacterial infections Related to the potency of preparation and duration of treatment; skin of face is more susceptible. Conventionally, agents used on living surfaces (skin, mouth) are called antiseptics while those used for inanimate objects (instruments, privies, water supply) are called disinfectants. While sterilization means complete killing of all forms of microorganisms, disinfection refers to reduction in the number of viable pathogenic microbes to a level that they do not pose a risk to individuals with normal host defence. The era of antiseptics and disinfectants was heralded by Semmelweiss (washing of hands in chlorinated lime) and Lister (antiseptic surgery by the use of phenol) in the 19th century. However, many systemic antimicrobials are applied topically as well, and some antibiotics (bacitracin, neomycin) are restricted to topical use, but are generally not enumerated with the antiseptics. A disinfectant in addition should not corrode or rust instruments and be easily washable. Spectrum of activity of majority of antisepticdisinfectants is wide, reflecting nonselectivity of action. Mechanisms of action of germicides are varied, but can be grouped into: (a) Oxidation of bacterial protoplasm. It is a general protoplasmic poison, injuring microbes and tissue cells alike- at higher concentrations causes skin burns and is a caustic. Organic matter diminishes its action slightly while alkalies and soaps do so profoundly (carbolic soaps are not more germicidal than soap itself). Hexylresorcinol It is a more potent derivative of the phenolic compound resorcinol that is odourless and nonstaining; used as mouthwash, lozenge and as antifungal. Therapeutic index of an antiseptic is defined by comparing the concentration at which it acts on microorganisms with that which produces local irritation, tissue damage or interference with healing. Quaternary ammonium (Cationic): Cetrimide, Benzalkonium chloride, Dequalinium chloride. Metallic salts: Silver nitrate, Silver sulfadiazine, Mild silver protein, Zinc sulfate, Calamine, Zinc oxide. Chloroxylenol It has a phenol coefficient of 70; does not coagulate proteins, is noncorrosive, nonirritating to intact skin, but efficacy is reduced by organic matter. Hexachlorophene this chlorinated phenol acts by inhibiting bacterial enzymes and (in high concentration) causing bacterial lysis. Use of a 3% solution for baby bath markedly reduced the incidence of staphylococcal infections, but produced brain damage (especially in premature neonates). The available oxygen and germicidal capacity is used up if much organic matter is present-the solution gets decolourised. The action is rather slow and higher concentrations cause burns and blistering-popularity therefore has declined. It has also been used to disinfect water (wells, ponds) and for stomach wash in alkaloidal poisoning (except atropine and cocaine which are not efficiently oxidized). Hydrogen peroxide It liberates nacent oxygen which oxidizes necrotic matter and bacteria. Catalase present in tissues speeds decomposition resulting in foaming-helps in loosening and removing slough, ear wax, etc. Some individuals are sensitive to iodine-rashes and systemic manifestations occur in them.
When breathing enters the consciousness and produces discomfort, it is called dyspnea. The normal person is not aware of any increase in breathing until the pulmonary ventilation is doubled. The pathological conditions when dyspnea occurs are: Chapter 127 t Disturbances of Respiration 731 1. Respiratory Disorders Dyspnea occurs in the respiratory disorders, characterized by mechanical or nervous hindrance to respiratory movements and obstruction in any part of respiratory tract. Cardiac Disorders Dyspnea is common in left ventricular failure and decompensated mitral stenosis. Metabolic Disorders Metabolic disorders, which cause dyspnea are diabetic acidosis, uremia and increased hydrogen ion concentration. Gradual increase followed by gradual decrease in force of respiration is called waxing and waning of breathing. Apneic period When, the force of breathing is reduced to minimum, cessation of breathing occurs for a short period. Causes for Waxing and Waning Initially, during forced breathing, large quantity of carbon dioxide is washed out from blood. When partial pressure of carbon dioxide decreases, respiratory centers become inactive. Now, the respiratory centers are activated, there is accumulation of carbon dioxide resulting in gradual increase in the force of breathing. Causes of Abrupt Apnea and Hyperpnea Due to apnea, carbon dioxide accumulates and it stimulates the respiratory centers, leading to hyperventilation. Conditions when Biot Breathing Occurs Biot breathing does not occur in physiological conditions. It occurs in conditions involving nervous disorders due to lesions or injuries to brain. During deep sleep In high altitude After prolonged voluntary hyperventilation During hibernation in animals In newborn babies After severe muscular exercise. Quantity of reduced hemoglobin should be at least 5 to 7 g/dL in the blood to cause cyanosis. These areas are lips, cheeks, ear lobes, nose and fingertips above the base of the nail. Cyanosis does not occur in anemic hypoxia because the hemoglobin content itself is less. Due to poisoning, hemoglobin is altered into methemoglobin or sulfhemoglobin, which causes cyanosis. The cyanotic discoloration is due to the dark color of these compounds only and not due to reduced hemoglobin. So the quantity of deoxygenated blood increases, which causes bluish discoloration of skin. Presence of carboxyhemoglobin decreases the release of oxygen from hemoglobin and the oxygen-hemoglobin dissociation curve shifts to left. It is still more dangerous because, during carbon monoxide poisoning, the partial pressure of oxygen in blood may normal in spite of low oxygen content of blood. So, the regular feedback stimulation of respiratory centers by hypoxia does not take place because of normal partial pressure of oxygen. However, low oxygen content in blood affects the brain, resulting in unconsciousness. While breathing air with 1% of carbon monoxide, saturation of hemoglobin with carbon monoxide becomes 15% to 20%. While breathing air containing carbon monoxide more than 1%, the saturation becomes 30% to 40%. Open Pneumothorax After the injury, an open communication is developed between pleural cavity and exterior. When a large portion of lung is collapsed, the partial pressure of oxygen is reduced in blood, leading to respiratory disturbances. It causes collapse of lungs due to increased surface tension, which leads to respiratory distress syndrome.