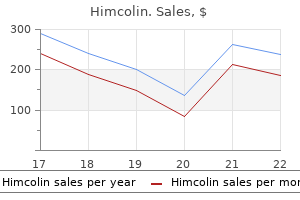
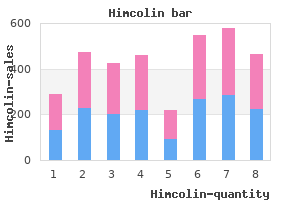
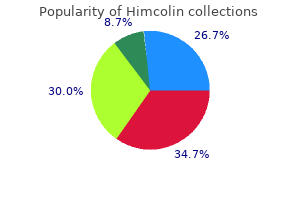
"Himcolin 30 gm mastercard, erectile dysfunction drugs stendra".
Y. Nefarius, M.B. B.CH. B.A.O., M.B.B.Ch., Ph.D.
Clinical Director, Roseman University of Health Sciences
However, biodegradation data derived from use of a pure culture are not strictly relevant to natural waters that contain mixed cultures. Mixed microorganisms in sewage sludge or activated sludge acclimated to cyanide also significantly biodegrade concentrations 100 mg/L of most simple and complex cyanides (Gaudy et al. These data may represent a biodegradation half-life; however, the possibility of loss by chemical reaction was not addressed in this study. It is known that there is a natural attentuation of the cyanide ion and thiocyanide concentrations in waste waters, for example those obtained gold mill tails, that is due the acclimation of indigenous microflora in the tailings (Akcil and Mudder 2003; Oudjehani et al. A number of microorganisms have been identified that are capable of uptake, conversion, sorption, and/or precipitation of the cyanide ion, cyanate, and thiocyanate, including species of the genera, Actinomyces, Alcaligenes, Arthrobacter, Bacillus, Micrococcus, Neisseria, Paracoccus, Pseudomonas, and Thiobacillus (Akcil and Mudder 2003). Some of these species, for example Pseudomonas, are capable of using the cyanide ion and thiocyanate as the sole source of carbon and nitrogen and therefore, are particularly effective at cyanide degradation. In fact, Pseudomonas is the basis of commercial applications for degrading the cyanide ion to ammonia and carbonate in waste waters generated in mining operations that use the cyanide ion to leach gold and other precious metals for low-grade ores (Akcil and Mudder 2003). Raybuck (1992) has recently reviewed the role of microbes in cyanide degradation and has categorized the microbial enzymes that use the cyanide ion as a substrate according to the following types of reactions: substitution/addition, hydrolysis, oxidation, and reduction. Sulfur transferases such as rhodanese are involved in substitution reactions that result in the conversion of the cyanide ion to the less toxic thiocyanate, whereas pyridoxal phosphate enzymes are involved in substitution/addition reactions that result in production of nitrile derivatives of -amino acids. These organic nitriles may then be ultimately degraded via enzyme catalyzed hydrolysis to either the corresponding amino acid and ammonia (without formation of the free amide) or the carboxylic acid and ammonia (via formation of the free amide). The cyanide hydratase and cyanidase enzymes catalyze the hydrolysis of the cyanide ion to formamide or formic acid and ammonia, respectively. Thus, these hydrolytic systems are some of the most promising for detoxification of cyanide-containing waste waters (Raybuck 1992). Bacillus pumulus, Pseudomonas fluorescens, and Pseudomonas paucimobili have all been found to oxidize the cyanide ion to ammonia and carbon dioxide (Meyers et al. In an aerobic batch bioreactor experiment, Pseudomonas putida was found to significantly degrade 4 mM sodium cyanide (cyanide concentration approximately 100 mg/L) to ammonia and carbon dioxide (Chapatwala et al. Other evidence indicates that formamide and formate are additional transformation products in microbial oxidation of the cyanide ion by this species, inferring that there may be more than one pathway of cyanide biotransformation involved (Kunz et al. Several bacterial species have been identified that are capable of oxidative degradation of metallocyanides (Silva-Avalos et al. The cyanide oxygenase system involved in this process offers a new technology for the treatment of metal cyanide wastes (Raybuck 1992). The ferrocyanide complex is not easily biodegradable (Belly and Goodhue 1976; Pettet and Mills 1954). However, when an aqueous solution of potassium ferrocyanide was seeded with pure culture of Pseudomona aeruginosa, or E. It was shown that the free cyanide formation was due to biodegradation and not to either photolysis or hydrolysis. The relevance of this study to the fate of ferrocyanide complexes in natural water or industrial effluents is difficult to assess because ferrocyanide concentrations used in these experiments (3,300 mg/L) are rarely encountered in these media. Biodegradation is also a significant transformation process for thiocyanates in natural waters; however, additional data are needed to assess the relative importance of this process. Like the cyanide ion, thiocyanate is toxic to microorganisms at high concentrations and acclimated cultures have increased tolerance to this compound (Boucabeille et al. Thiosulfate ion (S2O3-2) was identified as the intermediate in this degradation pathway. Several studies document the biodegradation of mixtures of cyanides and thiocyanate in waste waters. Complete biodegradation of simple and metal complexed cyanides and thiocyanate from mining waste waters by various bacteria belonging to the families Pseudomonadaceae, Vibrioniaceae, and Enterobacteriaceae has recently been reported (Boucabeille et al. Biodegradation of cyanide and thiocyanate resulted in the formation of ammonia, with or without accumulation of nitrite and/or nitrate, depending on whether a batch, fed-batch, or continuous treatment process was used. Cyanogen chloride also hydrolyzes slowly to cyanic acid and hydrochloric acid in water at pH 7, with a rate constant of 6.
Gene editing does not typically result in gene dysregulation, and no other region of the genome is likely to be affected (8,9). This gene correction strategy relied on the ability to deliver a functional gene along with other related elements needed to promote sustained, high-level gene expression. The drawbacks of this approach included loss of physiological regulation of the treated gene, and disruption and possible dysregulation of other genes. Even with this unfortunate event, the overall outcome of the trial provided evidence that gene therapy is equivalent or superior to the previous standard of care (hematopoietic cell transplantation), providing superior immune function, improved disease-free survival, and a better quality of life (5,6,10,11). It is important to note that the effects of insertional mutagenesis may vary from patient to patient. It can take a long time for side effects to occur, as demonstrated by the gene therapy trials performed to date. Stem Cell Therapy Stem cell therapy vectors Traditionally, stem cell therapy has entailed the use of bone marrow cells; this method has been experimentally and clinically proven in many thousands of successful bone marrow transplants over the last 50 years. While embryonic stem cells provide an opportunity to understand more deeply how stem cells work, their use remains controversial and various biological and legal constraints prevent their therapeutic use. More relevant to clinical care are induced pluripotent stem cells, which are embryonic stem cell-like cells from the skin or blood of adults that have been engineered with the potential to develop into any other type of cell in the body. Good to Know Pluripotent stem cells are cells capable of developing into almost any type of cell in the body. Hematopoietic stem cell transplantation usually uses stem cells from the bone marrow or umbilical cord blood of a matched donor. These cells are thought to be located in the walls of the blood vessels and to perform key functions, such as supporting hematopoietic stem cells in the bone marrow and modulating the immune response. Methods of stem cell therapy There are at least two methods of cell therapy: traditional hematopoietic stem cell transplantation and immunomodulation. Traditional hematopoietic stem cell transplantation involves replacing the entire blood-producing system of the recipient patient with that of a healthy donor. Stem cells, for example mesenchymal stromal cells, can also play a role in tissue repair and healing after injury. Side effects of stem cell therapy the most notable side effect of stem cell therapy is tumorigenesis, or the uncontrolled growth of stem cells, which can give rise to benign or malignant tumors. Most cancers are thought to originate from so-called "cancer stem cells," which are in many ways similar to normally functioning stem cells in their cellular processes and metabolic pathways. Because of this, some donor stem cells potentially can cause malignancies in the patient; indeed, donor263 Fanconi Anemia: Guidelines for Diagnosis and Management derived leukemias have been reported in some recipients of hematopoietic cell transplantation. Multiple researchers have observed this phenomenon in animal models when mesenchymal stromal cells were transplanted from one organism to another and gave rise to cancers (26). In theory, additional side effects are possible because of the specific functions of stem cells. Stem Cell Gene Therapy An effective gene therapy strategy must target the cell type relevant to the specific disease. For this reason, many gene therapies have attempted to deliver genes to stem cells. It seems only logical that the parallel tracks of gene therapy and stem cell therapy should be joined in one concerted effort termed "stem cell gene therapy. For reasons mentioned above, the leading strategy for gene therapy represents a shift away from gene addition, in which an entirely new gene is pasted into the genome with the help of viruses or transposons, and a move toward genome editing, whereby the pathogenic mutation is corrected in its natural gene location with the aid of newly engineered molecules called zincfinger nucleases, transcription activator-like effector nucleases, or homing endonucleases. In this fashion, the pathogenic mutation is permanently changed to the normal sequence. One of the advantages of gene editing is its spectacular flexibility and range of use; it can be used for targeted delivery, tissue-specific regulatory sequences, or transduction of cell types committed to tissue-specific differentiation programs. The great early promise of stem cell gene therapy comes-as with many advances in medicine-with some risk. Viral transduction, however, resulted in transient or no correction of hematopoietic cells, an observation consistent with only short-term functional gene complementation (27-30). Individuals with a human leukocyte antigen-matched sibling donor, an abnormal karyotype, or a serious infection are not eligible for the trial (3,31,32).
Other commenters recommended reducing the weight of the Efficiency and Cost Reduction domain and increasing the weight of the Person and Community Engagement domain. Response: We thank the commenter for its input, and will take this recommendation into consideration for future years of the program as we continue evaluating our domain weighting policies. Therefore, previously adopted minimum numbers of cases for those measures are also set forth in the table below. Minimum number of cases Hospitals must report a minimum of three eligible cases on any one underlying indicator. In the same rule, we finalized a schedule for all future baseline and performance periods for previously adopted measures. Efficiency and Cost Reduction: *** Baseline period · January 1, 2020December 31, 2020. The performance standards must include levels of achievement and improvement, as required by section 1886(o)(3)(B) of the Act, and must be established no later than 60 days before the beginning of the performance period for the fiscal year involved, as required by section 1886(o)(3)(C) of the Act. In addition, when establishing the performance standards, section 1886(o)(3)(D) of the Act requires the Secretary to consider appropriate factors, such as: (1) Practical experience with the measures, including whether a significant proportion of hospitals failed to meet the performance standard during previous performance periods; (2) historical performance standards; (3) improvement rates; and (4) the opportunity for continued improvement. As a result, the previously finalized performance standards for those measures are not included in this table. The Consistency Points take into consideration the scores of all eight Person and Community Engagement dimensions. The previously adopted performance standards for these measures are set out in the table below. Mean of the lowest decile Medicare Spending per Beneficiary ratios across all hospitals during the performance period. Specifically, the Achievement Threshold and Benchmark values, while accurate, were presented in the wrong categories. As a result, the previously finalized performance standards for those three measures are not included in this table. For this reason, we did not include proposed performance standards for this measure in the proposed rule. The previously adopted and newly displayed performance standards for the other measures are also set out in the table below. Median Medicare Spending per Beneficiary ratio across all hospitals during the performance period. These newly proposed performance standards for these measures are set out in the table below. By publicly reporting quality data, we strive to put patients first by ensuring they, along with their clinicians, are empowered to make decisions about their own healthcare using information aligned with meaningful quality measures. We believe this framework will allow hospitals and patients to continue to obtain meaningful information about hospital performance and incentivize quality improvement while also streamlining the measure sets to reduce duplicative measures and program complexity so that the costs to hospitals associated with participating in these programs does not outweigh the benefits of improving beneficiary care. By including these measures in the Program, we seek to encourage hospitals to address the serious harm caused by these adverse events and to reduce them. Over time, the list has been expanded to signify adverse events that are unambiguous (clearly identifiable and measurable), serious (resulting in death or significant disability), and usually preventable. The list has been revised since then, most recently in 2011, and now consists of 29 events grouped into 7 categories: Surgical, product or device, patient protection, care management, environmental, radiologic, and criminal. We also sought public comment on confidential reporting and future public reporting of some of our measures stratified by patient dual eligibility. With regard to value-based purchasing programs, commenters also cautioned to balance fair and equitable payment while avoiding payment penalties that mask health disparities or discouraging 258 Available at. Furthermore, we continue to consider options to address equity and disparities in our value-based purchasing programs. We take this feedback seriously and will continue to review social risk factors on an on-going and continuous basis. In addition, we both welcome and appreciate stakeholder feedback as we continue our work on these issues. These previously finalized measures, with 41475 their full measure names, are shown in the table below.
Post-Marketing: Reported side effects from use outside the United States include hypersensitivity/anaphylactic reactions, viral transmission. If appropriate and if symptoms subside, infusion may be resumed at a tolerated rate. Invert the bottle of diluent and rapidly insert the other end of the transfer needle into the slightly angled C1 inhibitor vial. Do not use if turbid or discolored (should be colorless to slightly blue and clear). Attach a filter needle to a 10-mL syringe and withdraw 500 units (5 mL) from each vial (total dose 1,000 units in 10 mL [100 units/mL]). C1 inhibitor is a normal constituent of human blood; a serine proteinase inhibitor. It regulates activation of the complement and intrinsic coagulation (contact system) pathway and regulates the fibrinolytic system. One unit of C1 inhibitor corresponds to the amount of C1 inhibitor present in 1 mL of normal fresh plasma. Administer in a facility capable of monitoring the patient and responding to any medical emergency. Patient Education: Inform patient of risks for infectious agent transmission and of safety precautions taken during the manufacturing process. Maternal/Child: Category C: safety and effectiveness for use during pregnancy, labor, and delivery not established; use only if clearly needed. The most common side effects reported include headache, rash, sinusitis, and upper respiratory tract infections. Hypersensitivity reactions, including anaphylaxis, hives, hypotension, tightness of the chest, urticaria, and wheezing, have occurred. Interrupt or discontinue injection if indicated until symptoms subside, then resume at a tolerated rate. Given in combination with oral prednisone 10 mg administered daily throughout cabazitaxel treatment. Recommendations for dose adjustment for adverse reactions are listed in the following chart. Recommended Cabazitaxel Dose Modifications for Adverse Reactions Toxicity* Dose Modification Delay treatment until neutrophil count is. Delay treatment until improvement or resolution, then reduce dose of cabazitaxel to 20 mg/M2. Step 1: Initially withdraw the entire contents of the provided diluent and insert into the vial of cabazitaxel (60 mg/1. When transferring the diluent, direct the needle onto the inside wall of the cabazitaxel vial to limit foaming. Gently mix by repeated inversions for at least 45 seconds to ensure full mixing of drug and diluent. This initially diluted solution should be used immediately but must be used within 30 minutes of entry into the vial. Storage: Before use, store at 25° C (77° F), with excursions permitted between 15° to 30° C (59° to 86° F). Initial diluted solution should be used immediately but must be used within 30 minutes; discard any unused portion. Discard if either the initial diluted solution or final solution is not clear or appears to have precipitation. A microtubule inhibitor, it binds to tubulin and promotes its assembly into microtubules while simultaneously inhibiting disassembly. This leads to the stabilization of microtubules, which results in the inhibition of mitotic and interphase cellular functions. It is active in docetaxel-sensitive tumors and has demonstrated activity in tumor models insensitive to chemotherapy, including docetaxel. Mainly excreted in feces as numerous metabolites with minimal excretion of unchanged drug in urine. Treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen.