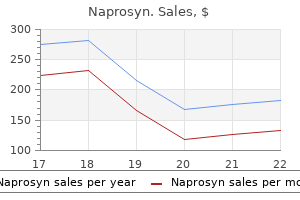
Parveen Kumar, CBE, BSc, MD, DM (HC), FRCP, FRCP(Edin)
- Professor of Medicine & Education, Barts and The London School of Medicine and Dentistry, Queen Mary, University of London, and Honorary Consultant Physician
- Gastroenterologist, Barts and The London Hospitals NHS Trust and Homerton Hospital NHS Foundation Trust, London, UK
A general equation (the Ng equation) has been proposed to describe the decomposition process (equation 29) arthritis treatments in dogs cheap 250mg naprosyn fast delivery. To speed up the degradation so that the quantity degraded turns into quantifiable in a shorter time period rheumatoid arthritis xerostomia purchase naprosyn no prescription, elevated temperatures are used arthritis in neck and pinched nerve cheap naprosyn 500mg amex, and the quantity of degradation is often calculated using the Arrhenius equation rheumatoid arthritis gout purchase naprosyn 250mg without prescription. The assumption made throughout these studies is that the mechanism of degradation is constant over a wide temperature vary. Furthermore, many compounds that exist as hydrates dehydrate at higher temperatures, which can change the degradation mechanism in the solid state. Excess water: this is an amount of water equal to or higher than the amount of moisture essential to dissolve the drug. In phrases of crystallinity, it should be famous that amorphous materials are generally much less secure than the corresponding crystalline part. For amorphous solids the web effect of water sorption is to lower the Tg and therefore plasticize the material. In flip this increases molecular mobility and due to this fact increases the potential for chemical reactivity (Ahlneck and Zografi, 1990). In prenomination studies a useful protocol to assess the effects of those components is as follows: the compound is precisely weighed into every of six open glass vials. Typically supplies would be sampled at common intervals up to a three-month time level to decide its stability. Photostability As illustrated in the e-book Drugs Photochemistry and Photostability, edited by Albini and Fasani (1998), a variety of drug types can endure photochemical degradation. However, instability as a result of gentle will in all probability solely be of concern if it considerably absorbs gentle with a wavelength higher than 330 nm and, even then, only if the response proceeds at a major price (Albini and Fasani, 1998). Light instability is an issue in each the stable and answer state and if highlighted would imply that formulations due to this fact have to be designed to defend the compound from its deleterious effects. The variety of compounds showing photo-instability is massive; for instance, Tnnesen (2001) has acknowledged that more than a hundred of essentially the most generally used medicine are unstable with respect to gentle. There are a selection of chemical teams that may be expected to give rise to decomposition. These embody the carbonyl group, the nitroaromatic group, the N-oxide group, the C�C bond, the aryl chloride group, groups with a weak C�H bond, and sulfides, alkenes, polyenes, and phenols (Albini and Fasani, 1998). This states that photostability testing ought to encompass pressured degradation and confirmatory testing. In addition, changes in bodily properties such as appearance and clarity or color ought to be noted. Light sources for testing the photostability embody synthetic daylight tubes, xenon lamps, tungstenmercury lamps, laboratory gentle, and natural light (Anderson et al. According to Aman and Thoma (2003), pure gentle varies between 389 and 500 W/m2 on a sunny day and 50 and a hundred and twenty W/m2 on a cloudy day. In terms of the kinetics, mild degradation in dilute solution is first order; however, in more concentrated solution decomposition approaches pseudo�zero order (Connors et al. The cause for this observation is that as the solution becomes more concentrated, degradation turns into limited due to the limited variety of incident quanta and quenching reactions between the molecules. It should be famous that ionizable compounds, for example, ciprofloxacin, confirmed large differences in photostability between the ionized and unionized types (Torniainen et al. The first occurred on the floor, which was adopted by a gas phase mass transfer step. After this the reaction proceeded by diffusion via a porous-reacted zone and chemical response at the boundary. Investigations by Aman and Thoma (2002) into the sunshine stability of nifedipine and molsidomine showed that particle dimension had a considerable impact on their photostability. It was found that after two hours of irradiation, decomposition was roughly 5% to 10% larger in the smaller-size ranges of the compounds. Solution Calorimetry Solution calorimetry supplies a direct measure of the thermodynamics (and kinetics) of dissolution. On mixing a fabric with an applicable solvent, heat circulate is measured (Royall and Gaisford, 2005) as a function of time and integrated to give the molar enthalpy of answer (DsolH). The cumulative enthalpy of solution encompasses a measure of the power associated with wetting, breaking of lattice bonds, and solvation. Classification of thermodynamic stability by solution calorimetry depends upon the energetics of wetting and solvation throughout polymorphs to be fixed, thereby providing a measure of lattice "power" or power. While the part related to heat of solvation may be accurately regarded as constant between polymorphs, the warmth of wetting may vary as a operate of crystal behavior and changes in surface characteristics, thus giving rise to some variability. In the former approach, the heat change brought on by dissolution of the solute results in a change in temperature of the answer. This leads to a temperature-time plot from which the warmth of solution is calculated. By distinction, in isothermal solution calorimetry (where, by definition, the temperature is maintained constant) any heat change is compensated by an equal, but opposite, vitality change, which is then the heat of answer (Gu and Grant, 2001). Furthermore, microsolution calorimetry can be utilized with as little as 3 to 5 mg of compound. The relative stability of polymorphs can be investigated by assessing the magnitude and signal (endothermic/exothermic) of the enthalpy of dissolution. It has also been used to quantitate binary mixtures of three crystalline types of sulfamethoxazole (Guillory and Erb, 1985). Gu and Grant (2001) used isoperibol resolution calorimetry (in addition to solubility measurements) to estimate the transition temperature of the polymorphs of sulfamerzine at any one temperature. They validated their methodology by a bracketing technique for assessing the solution-mediated transition of the polymorphs around the predicted transition temperature. One downside commonly encountered with lipophilic drug molecules is round wettability in aqueous media. Poor wettability often leads to a broadening of the dissolution course of to such an extent that, in some cases, integration of the information to decide the enthalpy of dissolution turns into intractable. To deal with this drawback surfactants can be used, as exemplified by the answer calorimetry assessment of cimetidine polymorphs (Souillac et al. The surfactants sodium dodecyl sulfate (1% w/v) and polysorbate eighty (3% w/v) have been used at concentrations considerably above their crucial micelle concentrations (cmcs) to help the wetting process. Positive results were obtained with regard to wettability, and they have been able to demonstrate that form A of cimetidine was probably the most stable polymorph. Hendriksen (1990) used resolution calorimetry to investigate the crystallinity of calcium fenoprofen samples. Lattices with larger levels of disruption, conversely, gave lower heats of resolution. Coquerel (2006) has discussed the issue of structural purity and the range of analytical methods (including resolution calorimetry) used to assess the side of the organic strong state. Solution calorimetry can additionally be used to consider amorphous-crystalline compositions in binary mixtures. Intrinsic Dissolution During the preformulation stage, an understanding of the dissolution fee of a drug candidate is important, since this property of the compound is recognized as a significant factor concerned in drug bioavailability. Dissolution of a stable usually takes place in two levels: solvation of the solute molecules by the solvent molecules adopted by transport of those molecules from the interface into the bulk medium by convection or diffusion. The main factor that determines the dissolution fee is the aqueous solubility of the compound; nonetheless, other factors corresponding to particle measurement, crystalline state (polymorphs, hydrates, and so forth. Moreover, physical properties similar to viscosity and wettability also can affect the dissolution process. To do this it should be carried out in a big volume of dissolution medium, or there have to be some mechanism whereby the dissolution medium is consistently replenished by fresh solvent to mimic the dynamic in vivo state. Provided this situation is met, the dissolution testing is defined as going down under sink situations. The intrinsic dissolution fee is the dissolution rate of the compound underneath the situation of constant floor area. The rationale for using a compressed disk of pure materials is that the intrinsic tendency of the check material to dissolve could be evaluated with out formulation excipients. Preformulation Investigations 85 Intrinsic dissolution charges of compounds obtained from rotating disks have been theoretically reported by Levich.
The two different classes of lubricants are anti-adherent excipients www.arthritis in feet buy generic naprosyn line, which cut back the friction between the tablet punch faces and tablet punches arthritis pain that won't go away naprosyn 250mg low price, and die-wall lubricant excipients degenerative arthritis definition order 500mg naprosyn visa, which reduce the friction between the pill surface and the die wall during and after compaction to allow easy ejection of the tablet rheumatoid arthritis diet gluten purchase 250 mg naprosyn fast delivery. When two contacting solids are displaced relative to each other and parallel to the airplane of contact, the resistance to the motion is termed friction. Contacting surfaces initially contact at factors on the asperities of the two surfaces. For relative movement of the supplies to happen parallel to the contact area, the supplies must be sheared. The larger the shear strength of the supplies involved, the greater the drive that shall be required to produce movement. Die-wall lubricants work by decreasing the shear drive essential to promote movement. The stage of a lubricant required in a pill is formulation dependent and may be optimized utilizing an instrumented tablet machine. On a single punch machine, strain gauges or load cells on the lower punch can immediately measure the force required to eject the tablet. An different methodology of measuring lubrication is to measure the ratio of the utmost forces on the higher and decrease punches in the course of the compaction cycle (Ho �lzer and Sj�gren, 1978). On a single punch machine, the decrease punch stays stationary through the compaction part, and the compaction stress is exerted by the movement of the higher punch. If there were no resistance to the movement of particles within the die, the pressure transmitted by way of the powder mattress to the lower punch would be the same because the pressure utilized by the upper punch. In apply, the interparticle friction and friction between the particles and the die wall result in a decrease pressure being transmitted to the decrease punch. The ratio of the upper to decrease punch forces, termed the R-value, has been used as a measure of the lubricant efficacy. The ejection drive may be measured both by instrumenting the punch ideas or by positioning load cells or pressure gauges below the ejection cam. While these issues can be overcome by growing the extent of lubricant added, the purpose must be to use the minimum stage of lubricant required to produce a suitable product, for causes discussed later. Fluid lubricants work by separating shifting surfaces fully with a layer of lubricant. These are typically mineral oils or vegetable oils, they usually may be either added to the mix or applied on to the die wall via depraved punches. Tablets containing oily lubricants might have a mottled appearance due to uneven distribution. Low-melting-point lipophilic solids can even act as fluid lubricants as a end result of the heat generated on the die wall is adequate to trigger them to melt, forming a fluid layer, which solidifies on ejection. Fluid lubricants include stearic acid, mineral oils, hydrogenated vegetable oils, glyceryl behenate, paraffins, and waxes. Low-melting-point lubricants must be used with caution in tablets which might be to be movie coated. Lubricants can soften on the pill floor during the film-coating process, resulting in tablets with a pitted look (Rowe and Forse, 1983). Boundary lubricants work by forming a skinny, strong film on the interface of the die and the pill. Such lubricants should have low shear power of their very own and kind interparticulate films that resist wear and cut back floor put on. Magnesium stearate is essentially the most broadly used lubricant; it types dense, high-meltingpoint films between the powder and the die wall to reduce friction and has low shear power between its personal surfaces. Despite its popularity, which is a reflection of its excellent lubricant properties, the material is way from perfect, and has had problems associated with product consistency, its impact on tablet strength, and its hydrophobicity. As the lubrication exercise of the fabric is said to its floor area, substantial decreases in ejection drive are famous when using sources of Table 9 Lubricants and Their Usage Lubricant Boundary lubricants Magnesium stearate Calcium stearate Sodium stearyl fumarate Polyethylene glycol 4000 and 6000 Sodium lauryl sulfate Magnesium lauryl sulfate Sodium benzoate Fluid lubricants Light mineral oil Hydrogenated vegetable oils Stearic acid Glyceryl behenate Level required (%) 0. The lubricant exercise of magnesium stearate is said to its readiness to form movies on the die wall floor. While the energy decreases with growing mixing time for all the materials tested, the effect is far more marked for supplies that deform plastically. When plastic-deforming supplies are compressed, the film of magnesium stearate across the particles stays relatively intact, so the interparticulate bonds are primarily between magnesium stearate particles, which, by virtue of its lubricant properties, are inherently weak. Materials that compact by fragmentation are less delicate to the lubricant as a end result of the fragmentation process produces a selection of clean, uncontaminated surfaces which might be able to kind sturdy bonds. The hydrophobic surfaces created by magnesium stearate have been shown to scale back the rate of dissolution and bioavailability of several tablet formulations. To decrease this effect, the manufacturing course of ought to be designed to ensure that the lubricant is the final excipient to be added. When each lubricant and disintegrant are being added to a granulated formulation, the disintegrant ought to be blended with the granules prior to the addition of the lubricant to reduce the chance of forming a hydrophobic film around the disintegrant. The mixing time for the lubricant should be set as the minimum time required to produce the desired impact. Some materials have adhesive properties and might adhere to the punch surfaces throughout compression. This will initially present itself as sticking, with a movie forming on the surface of the tablets, resulting in dull tablet surfaces. A more extreme model of sticking is selecting, where strong particles from the pill persist with the punch surface. This will typically be evident in the intagliations on the tablet surface, resulting in poor definition of the surface markings. Load cells have been fitted to the edge of feed frames of rotary machines to monitor the force required to knock tablets off the decrease punch following ejection. In practice, sticking tends to be monitored throughout extended runs on pill machines. Materials that can be added include talc, maize starch, and microcrystalline cellulose. It is now the norm to be able to determine new merchandise due to their unique look achieved by a combination of shape, measurement, shade, and surface markings. The compression weight of a high-dose drug will normally be decided by the level of filler required to impart the appropriate compactibility on the formulation, the aim being to produce the smallest attainable pill for ease of swallowing. For low-dose medication, the quantity of filler added is set by the minimum acceptable measurement for a pill. The actual minimum acceptable measurement for a pill will differ between pharmaceutical companies however is usually within the region of 6 or 7 mm diameter for a round tablet, which might equate with a compression weight of eighty to one hundred mg. Early tablets had been produced in cylindrical dies with flat-faced, flat bevel-edged, or concave punches. While the overwhelming majority of tablets are nonetheless round, over the last 20 years, there was an increasing shift away from round to shaped tablets for brand spanking new products. After round tablets, the commonest presentation is the capsule-shaped tablet, generally referred to as a caplet. This shape is particularly in style for high-dose merchandise the place the compression weight is essentially high, because the elongated form makes the tablet simpler to swallow than spherical tablets of equal weight. Less predictable was the discovering that other elements of tablet design, such as the number and depth of scorelines, the degree of rounding of corners, and the hardness of the finish indicated by floor shine, appeared to play a task in determining how straightforward or hard to swallow a pill may be. When designing a tablet form, consideration must also be given to the processes following compression, such as coating and packaging. These processes rely upon tablets with the flexibility to transfer relative to one another, and if the tablet shape allows tablets to kind "structures". The coloring of tablets may be achieved either by incorporating a dye or pigment into the powder prior to compression, or by making use of a colored coat to the pill following compression. Coating methods, that are handled in the part "Tablet Coating," are likely to present a more uniform shade than inclusion of pigments in the compression mix however have the drawback of requiring an extra manufacturing process. Lakes are fashioned by adsorbing dyes onto the surface of an inert substrate, usually aluminum hydroxide. If the colorant is being added to a granulate, soluble dyes can be dissolved in the granulating fluid to guarantee a uniform mix. When adding colorants as solids, the mixing process must be Oral Solid Dosage Forms 395 very efficient to achieve a uniform colour with no mottling. The usual method of inclusion is to kind a premix with a portion of the powder mix, and to mill this prior to incorporating the remaining components. The drawback could be decreased by the suitable number of colors, mottling being much less evident with pastel shades. The insoluble pigments are inclined to be extra mild, stable, and less susceptible to fading than the soluble dyes.
A Kernel Method for Multi-Labelled Classification In: Proceedings of Advances in Neural Information Processing Systems arthritis in dogs statistics buy discount naprosyn 250 mg line, Volume 14; 2001 rheumatoid arthritis diet food list generic naprosyn 500mg without prescription. Mining coherent dense subgraphs across massive biological networks for practical discovery arthritis treatment kerala naprosyn 500 mg without prescription. Learning protein features from Bi-relational graph of proteins and performance annotations arthritis in flat feet effective naprosyn 250 mg. Fast integration of heterogeneous information sources for predicting gene perform with restricted annotation. Incorporating practical inter-relationships into protein perform prediction algorithms. The FunCat, a practical annotation scheme for systematic classification of proteins from whole genomes. Identifying useful modules in interplay networks by way of overlapping markov clustering. Function-function correlated multi-label protein perform prediction over interaction networks. Proceedings of the sixteenth International Conference on Research in Computational Molecular Biology; Berlin, German: Springer-Verlag; 2012. Identification of functional modules in protein complexes via hyperclique sample discovery. Proceedings of the 23rd International Joint Conference on Artificial Intelligence; Beijing, China 2013. A framework for incorporating useful interrelationships into protein operate prediction algorithms. Learning with native and international consistency In: Proceedings of Advances in Neural Information Processing Systems; Vancouver, Canada 2003. The drug discovery and development course of for a typical research-based pharmaceutical firm could be broken down into 5 distinct stages as described briefly under. At every stage, there shall be a number of actions working in parallel, with the general objective of discovering a candidate drug and creating it to market as effectively as attainable. It ought to be famous that different firms might use barely different terminology and perform some activities eventually, but the total process is essentially the same. Strategic Research Feasibility research are performed to demonstrate whether or not interfering in a selected biological mechanism has an impact that may be of therapeutic value. The strategic analysis of a particular company is usually guided by factors similar to its inherent analysis competence and experience, therapeutic areas of unmet medical need, and market potential/commercial viability. Companies typically wish to develop a portfolio of merchandise inside a particular therapeutic space to capture a segment of the market. By focusing on a selected therapeutic area, an organization can construct on its current experience and competence in all of its functions with the aim of turning into a number one company in that area. Exploratory Research Exploratory research is an investigation of the organic mechanism and identification of a "chemical or biological lead" that interferes with it. During the exploratory analysis stage, diverse compounds are screened for the specified biological exercise. The purpose is to find a chemical or molecular entity that interferes with the process and to present a useful probe of the underlying therapeutic problem. Traditionally, this has been achieved by the natural chemist synthesizing compounds one at a time for the biologist to take a look at in a linear fashion. Over the final 20 years, there has been a rapid growth in the technologies for creating very massive and numerous quantities of artificial and biosynthetic molecules and for testing giant numbers of activity in much less time. Combinatorial strategies have replaced traditional artificial approaches to generate many attainable mixtures rapidly for organic testing. Approaches to lead generation throughout exploratory analysis often depend on how much is already identified in regards to the therapeutic goal into consideration. Alternatively, in some circumstances, the only out there biochemical information could be the structure of a ligand for the enzyme. There have been recent advances to create various biopharmaceutical molecules for analysis, for example, through antibody engineering to produce anticancer treatments (Morrow, 2007). Protein and glycosylation engineering may be employed to generate antibodies with enhanced effector capabilities. The presence or absence of one sugar residue can lead to a two-orders-of-magnitude distinction within the ability to kill most cancers cells by antibody-dependent cell cytotoxicity, which may result in lowered dose and cost. Together with combinatorial chemistry and rational drug design, genomics is rapidly emerging as a helpful method to allow corporations to considerably enhance the variety of drug targets and enhance on candidate selection success. A variety of firms have seen the potential in defining affected person groups based mostly on their genotypes and are actually investing a lot of cash to acquire a clearer understanding of the genes that are important to drug motion. Personal medicine has been in improvement for the explanation that 1980s: "Personalized treatment" is the place the physician prescribes the most effective therapy for a affected person primarily based on his or her genetic profile, whereas personalised products involve drugs which are actually made for a person patient. Candidate Drug Selection the chemical or biological lead is used to generate specific chemical compounds with the optimal desired characteristics, for example, efficiency, specificity, period, safety, and pharmaceutical features. During the candidate drug selection stage, the molecular lead is optimized by testing a variety of chosen compounds in in vitro and in vivo (animal) research. The goal is to choose one or more candidate medicine for improvement with probably the most desired traits. Pharmacological traits would possibly include acceptable absorption, potency, period of action, and selectivity for the receptor or enzyme. Safety characteristics will usually include noncarcinogenicity, nonteratogenicity, nonmutagenicity, and basic nontoxicity. The potential for these traits could be predicted from comparatively short-term preclinical toxipharmacological animal studies and in vitro exams. Phase zero studies involve the administration of single, subtherapeutic dose of the model new drug candidate to a small variety of human topics (10�15) to collect preliminary data on the pharmacokinetics (how the body processes the drug) and pharmacodynamics (how the drug works within the body). A part zero research gives no information on safety or efficacy, but drug builders can perform these research to rank drug candidates to decide which to take ahead. They enable selections to be made primarily based on human information as an alternative of relying on animal data, which could be unpredictive and vary between species. The potential advantages of phase 0 studies are to help candidate drug choice by getting an perception into the human pharmacokinetics, but additionally to assist to establish the probably pharmacological dose and likewise the first dose for the subsequent section I study. They can also identify early failures and save the corporate costs of additional growth. A record of most popular traits for a compound intended for oral strong dosage kind improvement is given in Table 1. However, within the occasion of having a alternative from a variety of compounds all possessing comparable pharmacological and safety properties, there could also be a big advantage for formulation growth in selecting a compound with probably the most preferred pharmaceutical improvement properties. It is beneficial to conduct preformulation research and biopharmaceutics research on the candidate drug selection stage to determine essentially the most relevant physicochemical and biopharmaceutical properties of potential candidate medicine to help candidate selection. Biopharmaceutics is the examine of how the physicochemical properties of the candidate medicine, the formulation/delivery system, and the route of administration have an result on the speed and extent of drug absorption. Appropriate biopharmaceutical data generated at this stage can also be very important in directing the candidate choice course of and for future dosage form design throughout improvement. The benefits of providing preformulation and biopharmaceutics enter through the candidate drug choice stage, to characterize the candidate drug and provide helpful info to assist the selection of the optimal compound for pharmaceutical growth, are emphasised in chapters three and four. Generally, any pharmaceutical issues could be found earlier, earlier than the candidate drug reaches growth, and any implications for product design and growth thought of in advance. The objective is to achieve a seamless transition from analysis to development, versus the traditional "over-the-wall" method that many pharmaceutical corporations experience to their costs. In spite of all these potential advantages of early pharmaceutical involvement to candidate drug selection, there could additionally be a number of barriers within an organization, which might hinder this fashion of working. Distance between the research group and the development group ought to not likely be thought of a barrier, though this may be the case for teams on completely different continents with completely different cultures and languages. The important factor for success seems to be the event of a proper mechanism for interplay, supported by senior management within the firm. This typically takes the type of a joint project staff with common meetings to evaluate progress. However, there should still be a scarcity of appreciation of what enter or experience pharmaceutical improvement can provide on the candidate drug choice stage.
Many approaches could be taken to induce polymorphic changes to explore its incidence arthritis in dogs medication uk discount naprosyn 250mg with mastercard. These embody solution-mediated transitions similar to recrystallization and solution maturation research (Cardew and Davey arthritis in neck cause sore throat buy discount naprosyn 250mg on-line, 1985) and thermally induced (Giron arthritis natural remedies cheapest naprosyn, 1995) and mechanical/pressure-induced adjustments similar to those exhibited by chlorpropamide (Wildfong et al exercises hip arthritis relief order discount naprosyn online. Other solvent-free strategies of isolating polymorphs contain quenching from the molten liquid or gaseous state (sublimation experiments), as are used to isolate polymorphs of venlafaxine hydrochloride (Roy et al. The prevalence of polymorphism can also be explored utilizing computational methodology (Beyer et al. The basis of these approaches entails in silico technology of all believable crystal structures, that are subsequently ranked in order of calculated lattice energies. While the applicability has been demonstrated for small rigid constructions, there are numerous limitations within the wider use of this approach-in explicit for structures with significant conformational flexibility. Furthermore, the veracity of such approaches is dependent upon the standard of the pressure fields used to model thermodynamic and kinetic properties satisfactorily (Gavezzotti, 2002), which renders the current approaches relevant solely to a small subset of organic structures. Two polymorphs have been recognized, the place the conformers in each kind sterically drive the three-dimensional packing and subsequent hydrogen-bonding motif. Preformulation Investigations 37 stabilization of the lattice of each polymorph to a differing extent and therefore considerably completely different solubility properties. The polymorphism on this case, and therefore the completely different coloration, was instantly as a result of a variation within the molecular conformation, giving rise to completely different three-dimensional packing (Yu, 2002). Bhatt and Desiraju (2007) describe the case of polymorphism of omeprazole, which is due to tautomerism, whereby the crystalline phases of this molecule are stable options of the 2 tautomers existing in a steady composition range. From a collection of experiments where the compositions of the stable solutions were changed, the authors had been in a place to determine the stable kind. Furthermore, these investigations recognized numerous questions concerning the classification of tautomers as polymorphism or totally different compounds. It was proposed that distinctions ought to be made in terms of a structural panorama, which incorporates a number of solvated and nonsolvated variations of the same molecular species, rather than absolute structural assignments. As such, an acceptable strategy to optimize the scope for assessing the polymorph hypersurface must subsequently be employed to mirror this case. An initial evaluation into the propensity for polymorphism is achieved by assessing physical reproducibility of the early batches ready by medicinal chemists. Furthermore, the process analysis and growth chemists will also be working on the synthesis and crystallization of the compound (Kim et al. In each these conditions, a range of solvents and crystallization conditions could additionally be explored, thus permitting (potentially) a large space of the polymorphism hypersurface to be evaluated. Moreover, this technique allows (to a sure degree) an evaluation into the position that impurities play in both templating or prohibiting growth of particular physical varieties. Additionally, initial screens, often using a single largescale batch, are additionally conducted in parallel on a micro- or semi-microscale. There are a variety of literature reviews of high-throughput platforms which have been utilized in academia and business for solid-form screening (Storey et al. Crystallization of options of acetaminophen and sulfathiazole gave rise to the different polymorphs, relying upon the extent of pretreatment. However, a extra rationalized and systematic strategy will typically lead to a extra fruitful search. Indeed, Stahly (2007), in a observe of caution, has stated that these highthroughput methods should be used along side these more systematic methodologies already in place. The rationale being that high-throughput polymorph screening methodologies using evaporation from properly plates tend to induce crystallization of a larger variety of metastable phases. This was illustrated by Capes and Cameron (2007) who noticed that the metastable form of acetaminophen was preferentially nucleated on the fringe of the meniscus and was another explanation for the looks of metastable types from these experiments. While elevated effort has been expended into exploring the polymorphic hypersurface of compounds, Jarring et al. Rather, the trouble should be centered on finding these bodily forms that have a bonus from a efficiency, formulation, and largescale manufacturing perspective. For polymorph screening functions, Mirmehrabi and Rohani (2005) have proposed a systematic approach to solvent choice on the premise of the hydrogen-bonding propensities of the solute and the solvent molecules. This approach due to this fact allowed easy identification of appropriate isolation conditions for these polymorphs. Interconversion of Polymorphs In addition to recrystallization, answer maturation or slurry research may be exploited to induce physical form conversions. These experiments are largely thermodynamically (rather than kinetically) driven and end result within the conversion of less secure varieties into physical forms that are more thermodynamically secure beneath the slurry circumstances. The solvents which are used can profoundly affect the rate and extent of conversion. Furthermore, the conversion rate was found to be slower in solvents that had a higher H-bond acceptor potential. It was noted that the speed of answer agitation and temperature also affected the velocity of the conversion. The extra intense the agitation, the quicker the conversion, and because the relationship between these two types of sulfamerazine is enantiotropic, the speed of conversion to type I was higher at decrease temperatures and lower near the transition temperature (508C). This work also advised that a solubility of no less than eight mM was wanted to be sure that the transformation proceeded satisfactorily. As touched upon earlier, the role of impurities also can play a crucial role in the formation or conversion of polymorphic types. These studies confirmed that a reaction by-product from the ultimate hydrolysis stage might stabilize different polymorphic types of the compound depending on the concentration of this by-product. By using molecular modeling strategies, they were able to present that the by-product, ethamidosulfathiazole, influenced the hydrogenbonding community and therefore polymorphic type and crystal morphology. The presence of impurities also can inhibit solution-mediated phase transformations, and this may be of explicit concern for screening for polymorphs at an early stage when maybe much less pure supplies are available (Gong et al. Additionally, adjustments within the artificial regime during the development of the drug compound via growth can provide rise to considerably totally different impurities, which even in very minor quantities may have an result on the looks or inhibition of particular polymorphs. An increase in the conversion price, nevertheless, was attributed to numerous components, namely, (1) increasing the solubility, (2) decreasing the level of the impurity causing the problem (possibly by changing the artificial route), (3) pretreatment of the stable to reach maximum supersaturation, and (4) increased temperature. The most recent information gathered from 245 polymorph screens carried at a contract research group reported that 90% of the compounds exhibited "a number of and noncrystalline types," of which solely 50% have been polymorphic (Stahly, 2007). Essentially this describes the scenario whereupon nucleation of a more stable kind, the beforehand isolated, metastable, form could now not be made. For example, the orthorhombic polymorph of paracetamol previously prepared by crystallization from solution had proved elusive since it was first found in 1974. However, crystallizing a supersaturated resolution with seeds obtained from melt crystallization gave rise to a suitable laboratory scale method to obtain this metastable phase (Nichols and Frampton, 1998). Blagden and Davey (2003) tried to combine the impact of thermodynamics, kinetics, and nucleation, in conjunction with modeling techniques, to the number of the polymorphs. However their method, whereas exhibiting some success, highlighted the necessity for an enchancment within the prediction of solute-solvent interactions. In most industries in which polymorphism plays an necessary role in supplies and their properties, there are several business drivers for polymorph characterization and selection. Secondly, it is important to have as much of the polymorph "hypersurface" mapped to ensure that all plausible low-energy constructions, which may symbolize developable forms, are isolated and characterised. Information on structural relationships and the convenience of interconversion (exploring both kinetics and mechanisms) permit the selection of essentially the most optimum or developable type. Leading on from that is one other enterprise driver, which relates to intellectual property. On identifying all possible developable polymorphs, it is essential to have patent coverage to defend intellectual property. In the realm of prescribed drugs there have been several important polymorph litigation instances (Bernstein, 2002; Cabri et al. Polymorph Production Methods A number of protocols exploring both solvent-mediated and nonsolvent-induced polymorphism can be employed to ensure that as a lot of the polymorphism hypersurface can be mapped. Crystallization from completely different solvents underneath variable circumstances, for example, totally different agitation speeds and temperatures (as exemplified by Blagden et al. Therefore, you will need to screen quite a lot of solvents that cover a diversity of physicochemical parameters (Table 4). Although the solvent of crystallization may be crucial in producing a selected polymorph, Getsoian et al. Precipitation by, for instance, addition of an antisolvent to an answer containing the drug or by pH adjustment of options of weak acids or bases (Bosch et al. Capes and Cameron (2007) reported that a metastable kind was obtained from the periphery of an evaporating answer.
Purchase naprosyn 250 mg on-line. Arthritis Which Medications Will Ease The Symptoms?.